Abstract
Purpose of review
Routine screening for colorectal cancer (CRC) in adults > 50 years of age has led to overall reductions in CRC incidence and CRC-related mortality. Yet CRC incidence among young adults age < 50 continues to increase without a clear explanation. This review examines the changing epidemiology of CRC and emerging evidence regarding the influence of genetic and lifestyle factors on risk for colorectal neoplasia.
Recent findings
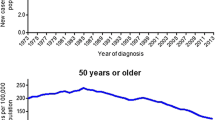
Young-onset CRC (yCRC), defined as CRC diagnosed in individuals younger than age 50, is a heterogeneous disease. Approximately, one in every five individuals affected with yCRC carries a pathogenic germline variant in genes associated with predisposition to cancer. However, most have no clinically identifiable risk factors. Analyses of birth cohorts estimate CRC risk among millennials to be 2–4 times higher than their grandparents’, suggesting that changes in health behaviors and environmental factors are having an impact on CRC risk. Young individuals with CRC tend to be diagnosed at later stages and often present with metastatic disease. yCRC tumors arise predominantly in the distal colon and are more likely than older-onset tumors to exhibit microsatellite and chromosome stable (MACS) phenotypes. Although yCRC patients are more likely than their older counterparts to be treated with multimodality chemotherapy regimens, more aggressive treatments have not yielded measurable survival gains. Since one in ten new CRC diagnoses involve individuals age < 50, recent guidelines have proposed lowering the age for average risk CRC screening from 50 to 45; however, further studies are needed to evaluate testing strategies based on individuals’ age and risk.
Summary
Significant shifts in CRC epidemiology and diversity of tumor phenotypes support genetic and environmental factors as modifiers of cancer risk. Emerging data correlating tumor molecular features with outcomes justify further investigation into mechanisms of carcinogenesis to elucidate how specific factors (inherited and/or acquired) might stimulate young-onset colorectal neoplasia.
Similar content being viewed by others
References and Recommended Readings
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cancer facts & figures 2018. Atlanta, Georgia: American Cancer Society2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-andstatistics/annual-cancer-facts-and-figures/2018/cancer-facts-and-figures-2018.pdf.
Colorectal cancer facts & figures 2017-2019. Atlanta, Georgia: American Cancer Society2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-andstatistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf.
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93. https://doi.org/10.3322/caac.21395.
Murphy CC, Singal AG, Baron JA, Sandler RS. Decrease in incidence of young-onset colorectal cancer before recent increase. Gastroenterology. 2018;155:1716–1719.e4. https://doi.org/10.1053/j.gastro.2018.07.045.
• Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal cancer incidence patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109(8):djw322. https://doi.org/10.1093/jnci/djw322 This analysis highlights the significant differences in CRC incidence across age groups as well as annual percentage change in incidence by age.
Bhandari A, Woodhouse M, Gupta S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: a SEER-based analysis with comparison to other young-onset cancers. J Investig Med. 2017;65(2):311–5. https://doi.org/10.1136/jim-2016-000229.
Sia CS, Paul E, Wale RJ, Lynch AC, Heriot AG, Warrier SK. No increase in colorectal cancer in patients under 50 years of age: a Victorian experience from the last decade. Color Dis. 2014;16(9):690–5. https://doi.org/10.1111/codi.12648.
Yeo H, Betel D, Abelson JS, Zheng XE, Yantiss R, Shah MA. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin Colorectal Cancer. 2017;16(4):293–9 e6. https://doi.org/10.1016/j.clcc.2017.06.002.
• Murphy CC, Lund JL, Sandler RS. Young-onset colorectal cancer: earlier diagnoses or increasing disease burden? Gastroenterology. 2017;152(8):1809–12 e3. https://doi.org/10.1053/j.gastro.2017.04.030 This study investigates the association between use of colonoscopy over the past two decades and its correlation with the increased incidence of yCRC. It finds that while initial rates of colonoscopy were increasing in parallel with the increasing incidence of yCRC, these two trends diverge around 2009 implying that increasing colonoscopy use does not explain the increasing yCRC incidence.
Murphy CC, Sanoff HK, Stitzenberg KB, Baron JA, Lund JL, Sandler RS. Patterns of sociodemographic and clinicopathologic characteristics of stages II and III colorectal cancer patients by age: examining potential mechanisms of young-onset disease. J Cancer Epidemiol. 2017;2017:4024580–10. https://doi.org/10.1155/2017/4024580.
Siegel RL, Miller KD, Jemal A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970-2014. JAMA. 2017;318(6):572–4. https://doi.org/10.1001/jama.2017.7630.
•• Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–81. https://doi.org/10.3322/caac.21457 This guideline recommended lowering the CRC screening age for asymptomatic average risk individuals from 50 to 45, generating debate.
Holowatyj AN, Ruterbusch JJ, Rozek LS, Cote ML, Stoffel EM. Racial/ethnic disparities in survival among patients with young-onset colorectal cancer. J Clin Oncol. 2016;34(18):2148–56. https://doi.org/10.1200/JCO.2015.65.0994.
You YN, Xing Y, Feig BW, Chang GJ, Cormier JN. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med. 2012;172(3):287–9. https://doi.org/10.1001/archinternmed.2011.602.
Davis DM, Marcet JE, Frattini JC, Prather AD, Mateka JJ, Nfonsam VN. Is it time to lower the recommended screening age for colorectal cancer? J Am Coll Surg. 2011;213(3):352–61. https://doi.org/10.1016/j.jamcollsurg.2011.04.033.
Myers EA, Feingold DL, Forde KA, Arnell T, Jang JH, Whelan RL. Colorectal cancer in patients under 50 years of age: a retrospective analysis of two institutions’ experience. World J Gastroenterol. 2013;19(34):5651–7. https://doi.org/10.3748/wjg.v19.i34.5651.
Jones HG, Radwan R, Davies M, Evans M, Khot U, Chandrasekaran TV, et al. Clinicopathological characteristics of colorectal cancer presenting under the age of 50. Int J Color Dis. 2015;30(4):483–9. https://doi.org/10.1007/s00384-015-2166-1.
Segev L, Kalady MF, Church JM. Left-sided dominance of early-onset colorectal cancers: a rationale for screening flexible sigmoidoscopy in the young. Dis Colon Rectum. 2018;61(8):897–902. https://doi.org/10.1097/DCR.0000000000001062.
Riaz R, Masood N, Benish A. Red flag symptoms: detailed account of clinicopathological features in young-onset colorectal cancer. Intest Res. 2017;15(2):203–7. https://doi.org/10.5217/ir.2017.15.2.203.
Chen FW, Sundaram V, Chew TA, Ladabaum U. Advanced-stage colorectal cancer in persons younger than 50 years not associated with longer duration of symptoms or time to diagnosis. Clin Gastroenterol Hepatol. 2017;15(5):728–37. e3. https://doi.org/10.1016/j.cgh.2016.10.038.
Fu J, Yang J, Tan Y, Jiang M, Wen F, Huang Y, et al. Young patients (≤ 35 years old) with colorectal cancer have worse outcomes due to more advanced disease: a 30-year retrospective review. Medicine (Baltimore). 2014;93(23):e135. https://doi.org/10.1097/MD.0000000000000135.
Silla IO, Rueda D, Rodriguez Y, Garcia JL, de la Cruz VF, Perea J. Early-onset colorectal cancer: a separate subset of colorectal cancer. World J Gastroenterol. 2014;20(46):17288–96. https://doi.org/10.3748/wjg.v20.i46.17288.
• Rho YS, Gilabert M, Polom K, Aladashvili A, Kopeckova K, Megdanova V, et al. Comparing clinical characteristics and outcomes of young-onset and late-onset colorectal cancer: an international collaborative study. Clin Colorectal Cancer. 2017;16(4):334–42. https://doi.org/10.1016/j.clcc.2017.03.008 This international, multicenter analysis looked at differences in use of adjuvant treatments for yCRC.
Chang DT, Pai RK, Rybicki LA, Dimaio MA, Limaye M, Jayachandran P, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol. 2012;25(8):1128–39. https://doi.org/10.1038/modpathol.2012.61.
Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. https://doi.org/10.1146/annurev-pathol-011110-130235.
Rodriguez-Salas N, Dominguez G, Barderas R, Mendiola M, Garcia-Albeniz X, Maurel J, et al. Clinical relevance of colorectal cancer molecular subtypes. Crit Rev Oncol Hematol. 2017;109:9–19. https://doi.org/10.1016/j.critrevonc.2016.11.007.
Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7. https://doi.org/10.1038/nature11252.
Ballester V, Rashtak S, Boardman L. Clinical and molecular features of young-onset colorectal cancer. World J Gastroenterol. 2016;22(5):1736–44. https://doi.org/10.3748/wjg.v22.i5.1736.
Banerjea A, Hands RE, Powar MP, Bustin SA, Dorudi S. Microsatellite and chromosomal stable colorectal cancers demonstrate poor immunogenicity and early disease recurrence. Color Dis. 2009;11(6):601–8. https://doi.org/10.1111/j.1463-1318.2008.01639.x.
Antelo M, Balaguer F, Shia J, Shen Y, Hur K, Moreira L, et al. A high degree of LINE-1 hypomethylation is a unique feature of early-onset colorectal cancer. PLoS One. 2012;7(9):e45357. https://doi.org/10.1371/journal.pone.0045357.
Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–57. https://doi.org/10.1056/NEJMoa022289.
Li P, Xiao ZT, Braciak TA, Ou QJ, Chen G, Oduncu FS. Impact of age and mismatch repair status on survival in colorectal cancer. Cancer Med. 2017;6(5):975–81. https://doi.org/10.1002/cam4.1007.
Schellerer VS, Merkel S, Schumann SC, Schlabrakowski A, Fortsch T, Schildberg C, et al. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer : CRC in patients under 50 years of age. Int J Color Dis. 2012;27(1):71–9. https://doi.org/10.1007/s00384-011-1291-8.
• Kneuertz PJ, Chang GJ, Hu CY, Rodriguez-Bigas MA, Eng C, Vilar E, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg. 2015;150(5):402–9. https://doi.org/10.1001/jamasurg.2014.3572 Analysis of hospital system data found yCRC patients were significantly more likely to receive high intensity multimodality chemotherapy without associated surival gains.
•• Manjelievskaia J, Brown D, Mc Glynn KA, Anderson W, Shriver CD, Zhu K. Chemotherapy use and survival among young and middle-aged patients with colon cancer. JAMA Surg. 2017;152(5):452–9. https://doi.org/10.1001/jamasurg.2016.5050 This study highlights differences in the treatment approaches for yCRC. It describes an increase in use of adjuvant chemotherapy for yCRC patients compared to oldercounterparts with no differences in outcomes. The authors propose a potential overuse of chemotherapy among young patients with CRC.
Puccini A, Lenz HJ, Marshall JL, Arguello D, Raghavan D, Korn WM, Weinberg BA, Poorman K, Heeke AL, Philip PA, Shields AF, Goldberg RM, Salem ME Impact of patient age on molecular alterations of left-sided colorectal tumors. The Oncologist 2018. https://doi.org/10.1634/theoncologist.2018-0117, theoncologist.2018, theoncologist.0117.
Gargalionis AN, Piperi C, Adamopoulos C, Papavassiliou AG. Histone modifications as a pathogenic mechanism of colorectal tumorigenesis. Int J Biochem Cell Biol. 2012;44(8):1276–89. https://doi.org/10.1016/j.biocel.2012.05.002.
Pearlman R, Frankel WL, Swanson B, Zhao W, Yilmaz A, Miller K, et al. Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal Cancer. JAMA Oncol. 2017;3(4):464–71. https://doi.org/10.1001/jamaoncol.2016.5194.
Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. American College of Gastroenterology ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110(2):223–62; quiz 63. https://doi.org/10.1038/ajg.2014.435.
Genetic/familial high-risk assessment: Colorectal. In: NCCN Clinical Practice Guidelines in Oncology. 2018. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. Accessed 11/3/2018 2018.
•• Stoffel EM, Koeppe E, Everett J, Ulintz P, Kiel M, Osborne J, et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology. 2018;154(4):897–905 e1. https://doi.org/10.1053/j.gastro.2017.11.004 This analysis of outcomes of germline sequencing in patients referred to a cancer genetics clinic found 1 in 5 yCRC patients had pathogenic germline variants in common CRC genes, many of whom reported no family history of CRC.
Rosato V, Bosetti C, Levi F, Polesel J, Zucchetto A, Negri E, et al. Risk factors for young-onset colorectal cancer. Cancer Causes Control. 2013;24(2):335–41. https://doi.org/10.1007/s10552-012-0119-3.
Hidayat K, Yang CM, Shi BM. Body fatness at an early age and risk of colorectal cancer. Int J Cancer. 2018;142(4):729–40. https://doi.org/10.1002/ijc.31100.
Botma A, Nagengast FM, Braem MG, Hendriks JC, Kleibeuker JH, Vasen HF, et al. Body mass index increases risk of colorectal adenomas in men with Lynch syndrome: the GEOLynch cohort study. J Clin Oncol. 2010;28(28):4346–53. https://doi.org/10.1200/JCO.2010.28.0453.
de Kort S, Masclee AAM, Sanduleanu S, Weijenberg MP, van Herk-Sukel MPP, Oldenhof NJJ, et al. Higher risk of colorectal cancer in patients with newly diagnosed diabetes mellitus before the age of colorectal cancer screening initiation. Sci Rep. 2017;7:46527. https://doi.org/10.1038/srep46527.
Cavestro GM, Mannucci A, Zuppardo RA, Di Leo M, Stoffel E, Tonon G. Early onset sporadic colorectal cancer: worrisome trends and oncogenic features. Dig Liver Dis. 2018;50(6):521–32. https://doi.org/10.1016/j.dld.2018.02.009.
Botma A, Vasen HF, van Duijnhoven FJ, Kleibeuker JH, Nagengast FM, Kampman E. Dietary patterns and colorectal adenomas in Lynch syndrome: the GEOLynch cohort study. Cancer. 2013;119(3):512–21. https://doi.org/10.1002/cncr.27726.
Hsu L, Jeon J, Brenner H, Gruber SB, Schoen RE, Berndt SI, et al. A model to determine colorectal cancer risk using common genetic susceptibility loci. Gastroenterology. 2015;148(7):1330–9. e14. https://doi.org/10.1053/j.gastro.2015.02.010.
Zheng J, Zhao M, Li J, Lou G, Yuan Y, Bu S, et al. Obesity-associated digestive cancers: a review of mechanisms and interventions. Tumour Biol. 2017;39(3):1010428317695020. https://doi.org/10.1177/1010428317695020.
Kim NH, Jung YS, Yang HJ, Park SK, Park JH, Park DI, et al. Prevalence of and risk factors for colorectal neoplasia in asymptomatic young adults (20-39 years old). Clin Gastroenterol Hepatol. 2018;17:115–22. https://doi.org/10.1016/j.cgh.2018.07.011.
Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. multi-society task force on colorectal cancer. Gastroenterology. 2017;153(1):307–23. https://doi.org/10.1053/j.gastro.2017.05.013.
Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, Garcia FAR, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016;315(23):2564–75. https://doi.org/10.1001/jama.2016.5989.
Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104(3):739–50. https://doi.org/10.1038/ajg.2009.104.
•• Peterse EFP, Meester RGS, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124(14):2964–73. https://doi.org/10.1002/cncr.31543 This analysis incoorporates contemporary CRC incidence data to model outcomes of CRC screening to justify offering screening for CRC at an earlier age.
Corley DA, Peek RM Jr. When should guidelines change? A clarion call for evidence regarding the benefits and risks of screening for colorectal cancer at earlier ages. Gastroenterology. 2018;155(4):947–9. https://doi.org/10.1053/j.gastro.2018.08.040.
Bretthauer M, Kalager M, Weinberg DS. From colorectal cancer screening guidelines to headlines: beware! Ann Intern Med. 2018;169(6):405–6. https://doi.org/10.7326/M18-1720.
Kastrinos F, Uno H, Ukaegbu C, Alvero C, McFarland A, Yurgelun MB, et al. Development and validation of the PREMM5 model for comprehensive risk assessment of Lynch syndrome. J Clin Oncol. 2017;35(19):2165–72. https://doi.org/10.1200/JCO.2016.69.6120.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Anand Venugopal declares that he has no conflict of interest.
Elena Stoffel declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Colon
Rights and permissions
About this article
Cite this article
Venugopal, A., Stoffel, E.M. Colorectal Cancer in Young Adults. Curr Treat Options Gastro 17, 89–98 (2019). https://doi.org/10.1007/s11938-019-00219-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-019-00219-4