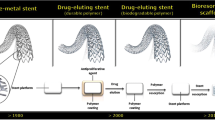
Opinion statement
Percutaneous coronary interventions will never become obsolete, as evolution is inherent to interventional cardiology. Current drug-eluting platforms have appreciably improved their safety and efficacy profiles in different clinical settings compared to first-generation devices such that it is difficult to consider other alternatives. However, there is definite biological plausibility to consider devices with bioabsorbable polymers and/or scaffolds. It is also an undeniable fact that many patients, based on variety of belief systems, would prefer not to have a permanently implanted device. BP DES with or without bioresorbable scaffolds offer advantages over durable polymer DES in restoring normal coronary physiology and vascular adaptive responses, resulting in late lumen gain and plaque regression. They will likely allow flexibility in treating complex CAD. However, so far, we have been able to prove non-inferiority in a selected population of patients without long-term data. Is “as good as” good enough? Are we ready to reach for the BRS or a BP DES in our catheterization laboratory based on preclinical and mechanistic data (endothelialization, OCT imaging, vasomotion) with limited human experience? I am not. While I will maximize my efforts to recruit patients in related randomized controlled trials, the technology is not ready for prime time. Randomized controlled trials are needed to determine whether any or all of these devices improve long-term outcome compared to best in class DP DES. Most definitive evidence is likely about a decade away. Until then, we can learn to be disciplined implanters not only in selecting the appropriate patient but also in perfecting implantation techniques


Similar content being viewed by others
Abbreviations
- ARC:
-
Academic Research Consortium
- BA:
-
Balloon angioplasty
- BMS:
-
Bare metal stent
- BRS:
-
Bioresorbable scaffold
- BP:
-
Bioabsorbable polymer
- CAD:
-
Coronary artery disease
- DAPT:
-
Dual anti-platelet therapy
- BVS:
-
Abbott Vascular-poly-l -lactic acid (PLLA)-based absorb bioresorbable vascular scaffold
- DP:
-
Durable polymer
- DP-CoCr-EES:
-
Durable polymer cobalt-chromium everolimus-eluting stent(s) - (Xience V and Promus, Boston Scientific Corp., Natick, Massachusetts)
- DP-PtCR-EES:
-
Durable polymer, platinum chromium everolimus-eluting stents (Promus Element, Boston Scientific Corp., Natick, Massachusetts)
- BP -PtCR-EES:
-
Bioabsorbable polymer, platinum chromium everolimus-eluting stents SYERGY, (Boston Scientific Corp., Natick, Massachusetts)
- DP CoCr-ZES:
-
Durable polymer cobalt-chromium zotarolimus eluting stents
- IVUS:
-
Intravascular ultrasonography
- LST:
-
Late stent thrombosis
- OCT:
-
Optical coherence tomography
- PVDF-HFP:
-
Polyvinylidene fluoride-co-hexafluoropropene
- PCI:
-
Percutaneous coronary intervention
- SES:
-
Sirolimus-eluting stents
- ST:
-
Stent thrombosis
- TLF:
-
Target lesion failure
- VLST:
-
Very late stent thrombosis
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bangalore S, Kumar S, Fusaro M, et al. Short- and long-term outcomes with drug-eluting and bare-metal coronary stents: a mixed-treatment comparison analysis of 117 762 patient-years of follow-up from randomized trials. Circulation. 2012;125:2873–91.
Windecker S, Stortecky S, Stefanini GG, et al. Revascularisation versus medical treatment in patients with stable coronary artery disease: network meta-analysis. BMJ. 2014;348:g3859.
Otsuka F, Pacheco E, Perkins LE, et al. Long-term safety of an everolimus-eluting bioresorbable vascular scaffold and the cobalt-chromium XIENCE V stent in a porcine coronary artery model. Circ Cardiovasc Interv. 2014;7:330–42.
Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2015;65:2496–507.
• Iqbal J, Serruys PW, Silber S, et al. Comparison of zotarolimus- and everolimus-eluting coronary stents: final 5-year report of the RESOLUTE all-comers trial. Circ Cardiovasc Interv. 2015;8, e002230. This study highlights that 1 in 5 patients with best in classs 2nd generation dug eluting stents will, within 5 years of follow up, have a MACE.
Farooq V, Serruys PW, Zhang Y, et al. Short-term and long-term clinical impact of stent thrombosis and graft occlusion in the SYNTAX trial at 5 years: synergy between percutaneous coronary intervention with taxus and cardiac surgery trial. J Am Coll Cardiol. 2013;62:2360–9.
Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16.
Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–84.
Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–35.
Kolandaivelu K, Swaminathan R, Gibson WJ, et al. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–9.
Otsuka F, Yahagi K, Ladich E, et al. Hypersensitivity reaction in the US Food and Drug Administration-approved second-generation drug-eluting stents: histopathological assessment with ex vivo optical coherence tomography. Circulation. 2015;131:322–4.
Lagerqvist B, Carlsson J, Frobert O, et al. Stent thrombosis in Sweden: a report from the Swedish coronary angiography and angioplasty registry. Circ Cardiovasc Interv. 2009;2:401–8.
Joner M, Finn AV, Farb A, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202.
Virmani R, Guagliumi G, Farb A, et al. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent: should we be cautious? Circulation. 2004;109:701–5.
Raber L, Wohlwend L, Wigger M, et al. Five-year clinical and angiographic outcomes of a randomized comparison of sirolimus-eluting and paclitaxel-eluting stents: results of the sirolimus-eluting versus paclitaxel-eluting stents for coronary revascularization LATE trial. Circulation. 2011;123:2819–28. 6 p following 28.
Raber L, Kelbaek H, Ostojic M, et al. Effect of biolimus-eluting stents with biodegradable polymer vs bare-metal stents on cardiovascular events among patients with acute myocardial infarction: the COMFORTABLE AMI randomized trial. JAMA : J Am Med Assoc. 2012;308:777–87.
Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–402.
Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ. 2013;347:f6625.
Raber L, Magro M, Stefanini GG, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012;125:1110–21.
Sarno G, Lagerqvist B, Frobert O, et al. Lower risk of stent thrombosis and restenosis with unrestricted use of ‘new-generation’ drug-eluting stents: a report from the nationwide Swedish coronary angiography and angioplasty registry (SCAAR). Eur Heart J. 2012;33:606–13.
Sabate M, Cequier A, Iniguez A, et al. Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomised controlled trial. Lancet. 2012;380:1482–90.
Van Dyck CJ, Hoymans VY, Bult H, et al. Resolute and Xience V polymer-based drug-eluting stents compared in an atherosclerotic rabbit double injury model. Catheter Cardiovasc Interv : Off J Soc Cardiac Angiography Interv. 2013;81:E259–E268
Joner M, Nakazawa G, Finn AV, et al. Endothelial cell recovery between comparator polymer-based drug-eluting stents. J Am Coll Cardiol. 2008;52:333–42.
Windecker S, Serruys PW, Wandel S, et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. 2008;372:1163–73.
Byrne RA, Kastrati A, Kufner S, et al. Randomized, non-inferiority trial of three limus agent-eluting stents with different polymer coatings: the intracoronary stenting and angiographic results: test efficacy of 3 limus-eluting stents (ISAR-TEST-4) trial. Eur Heart J. 2009;30:2441–9.
Smits PC, Hofma S, Togni M, et al. Abluminal biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent (COMPARE II): a randomised, controlled, non-inferiority trial. Lancet. 2013;381:651–60.
Natsuaki M, Kozuma K, Morimoto T, et al. Biodegradable polymer biolimus-eluting stent versus durable polymer everolimus-eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2013;62:181–90.
Pilgrim T, Heg D, Roffi M, et al. Ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for percutaneous coronary revascularisation (BIOSCIENCE): a randomised, single-blind, non-inferiority trial. Lancet. 2014;384:2111–22.
Kaiser C, Galatius S, Jeger R, et al. Long-term efficacy and safety of biodegradable-polymer biolimus-eluting stents: main results of the Basel stent kosten-effektivitats trial-PROspective validation examination II (BASKET-PROVE II), a randomized, controlled noninferiority 2-year outcome trial. Circulation. 2015;131:74–81.
Raungaard B, Jensen LO, Tilsted HH, et al. Zotarolimus-eluting durable-polymer-coated stent versus a biolimus-eluting biodegradable-polymer-coated stent in unselected patients undergoing percutaneous coronary intervention (SORT OUT VI): a randomised non-inferiority trial. Lancet. 2015;385:1527–35.
Saito S, Valdes-Chavarri M, Richardt G, et al. A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (Clinical Evaluation of New Terumo Drug-Eluting Coronary Stent System in the Treatment of Patients with Coronary Artery Disease) trial. Eur Heart J. 2014;35:2021–31.
•• Kereiakes DJ, Meredith IT, Windecker S, et al. Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II Randomized Trial. Circ Cardiovasc Intervent. 2015;8. Data from EVOLVE II trial led to FDA approval of the first bioabsorbable polymer-coated DES in the US with similar 1 year TLR rates to best in class DES.
Stefanini GG, Byrne RA, Serruys PW, et al. Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J. 2012;33:1214–22.
Windecker S, Haude M, Neumann FJ, et al. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: results of the randomized BIOFLOW-II trial. Circ Cardiovasc Interv. 2015;8, e001441.
de Waha A, Stefanini GG, King LA, et al. Long-term outcomes of biodegradable polymer versus durable polymer drug-eluting stents in patients with diabetes a pooled analysis of individual patient data from 3 randomized trials. Int J Cardiol. 2013;168:5162–6.
Eppihimer MJ, Sushkova N, Grimsby JL, et al. Impact of stent surface on thrombogenicity and vascular healing: a comparative analysis of metallic and polymeric surfaces. Circ Cardiovasc Interv. 2013;6:370–7.
Meredith IT, Verheye S, Dubois CL, et al. Primary endpoint results of the EVOLVE trial: a randomized evaluation of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent. J Am Coll Cardiol. 2012;59:1362–70.
Serruys PW, Luijten HE, Beatt KJ, et al. Incidence of restenosis after successful coronary angioplasty: a time-related phenomenon. A quantitative angiographic study in 342 consecutive patients at 1, 2, 3, and 4 months. Circulation. 1988;77:361–71.
Rathore S, Terashima M, Suzuki T. Late-acquired stent malapposition after sirolimus-eluting stent implantation following acute coronary syndrome: angiographic, IVUS, OCT and coronary angioscopic observation. J Invasive Cardiol. 2009;21:666–7.
Brodie BR, Pokharel Y, Garg A, et al. Very late hazard with stenting versus balloon angioplasty for ST-elevation myocardial infarction: a 16-year single-center experience. J Interv Cardiol. 2014;27:21–8.
Yamaji K, Kimura T, Morimoto T, et al. Very long-term (15 to 23 years) outcomes of successful balloon angioplasty compared with bare metal coronary stenting. J Am Heart Assoc. 2012;1, e004085.
Lane JP, Perkins LE, Sheehy AJ, et al. Lumen gain and restoration of pulsatility after implantation of a bioresorbable vascular scaffold in porcine coronary arteries. JACC Cardiovasc Interv. 2014;7:688–95.
Gogas BD, King 3rd SB, Samady H. Bioresorbable polymeric scaffolds for coronary revascularization: Lessons learnt from ABSORB III, ABSORB China, and ABSORB Japan. Glob Cardiol Sci Pract. 2015;2015:62.
Tanaka A, Ruparelia N, Kawamoto H, Latib A, Colombo A. Positive vessel remodeling and appearance of pulsatile wall motion at long-term follow-up after bioresorbable scaffold implantation in a chronic total occlusion. JACC Cardiovasc Interv. 2015;8:1635–7.
Serruys PW, Onuma Y, Garcia-Garcia HM, et al. Dynamics of vessel wall changes following the implantation of the absorb everolimus-eluting bioresorbable vascular scaffold: a multi-imaging modality study at 6, 12, 24 and 36 months. EuroIntervention : J EuroPCR Collab Work Group Interv Cardiol Eur Soc Cardiol. 2014;9:1271–84.
Haude M, Ince H, Abizaid A, et al. Safety and performance of the second-generation drug-eluting absorbable metal scaffold in patients with de-novo coronary artery lesions (BIOSOLVE-II): 6 month results of a prospective, multicentre, non-randomised, first-in-man trial. Lancet. 2016;387:31–9.
Serruys PW, Ormiston J, van Geuns RJ, et al. A polylactide bioresorbable scaffold eluting everolimus for treatment of coronary stenosis: 5-year follow-up. J Am Coll Cardiol. 2016;67:766–76.
Karanasos A, Simsek C, Gnanadesigan M, et al. OCT assessment of the long-term vascular healing response 5 years after everolimus-eluting bioresorbable vascular scaffold. J Am Coll Cardiol. 2014;64:2343–56.
Brugaletta S, Radu MD, Garcia-Garcia HM, et al. Circumferential evaluation of the neointima by optical coherence tomography after ABSORB bioresorbable vascular scaffold implantation: can the scaffold cap the plaque? Atherosclerosis. 2012;221:106–12.
Onuma Y, Serruys PW. Bioresorbable scaffold: the advent of a new era in percutaneous coronary and peripheral revascularization? Circulation. 2011;123:779–97.
•• Ellis SG, Kereiakes DJ, Metzger DC, et al. Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med. 2015;373:1905–15. Landmark study of the ABSORB stent, the first bioresorbable vascular scaffold that got FDA approval, which showed, compared to Xience stent, similar target lesion failure rates at 1 year in patients with noncomplex obstructive CAD.
Stone GW, Gao R, Kimura T, et al. 1-year outcomes with the absorb Bioresorbable scaffold in patients with coronary artery disease: a patient-level, pooled meta-analysis. Lancet. 2016;387:1277–89.
Serruys PW, Chevalier B, Dudek D, et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomised controlled trial. Lancet. 2015;385:43–54.
Puricel S, Arroyo D, Corpataux N, et al. Comparison of everolimus- and biolimus-eluting coronary stents with everolimus-eluting bioresorbable vascular scaffolds. J Am Coll Cardiol. 2015;65:791–801.
Capodanno D, Gori T, Nef H, et al. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention : J EuroPCR Collab Work Group Interv Cardiol Eur Soc Cardiol. 2015;10:1144–53.
Puricel S, Cuculi F, Weissner M, et al. Bioresorbable coronary scaffold thrombosis: multicenter comprehensive analysis of clinical presentation, mechanisms, and predictors. J Am Coll Cardiol. 2016;67:921–31.
Kraak RP, Hassell ME, Grundeken MJ, et al. Initial experience and clinical evaluation of the absorb bioresorbable vascular scaffold (BVS) in real-world practice: the AMC single centre real world PCI registry. EuroIntervention : J EuroPCR Collab Work Group Interv Cardiol Eur Soc Cardiol. 2015;10:1160–8.
Cassese S, Byrne RA, Ndrepepa G, et al. Everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: a meta-analysis of randomised controlled trials. Lancet. 2016;387:537–44.
Lipinski MJ, Escarcega RO, Baker NC, et al. Scaffold thrombosis after percutaneous coronary intervention with ABSORB bioresorbable vascular scaffold: a systematic review and meta-analysis. JACC Cardiovasc Interv. 2016;9:12–24.
Rizik DG, Hermiller JB, Kereiakes DJ. The ABSORB Bioresorbable vascular scaffold: a novel, fully resorbable drug-eluting stent: current concepts and overview of clinical evidence. Catheter Cardiovasc Interv : Off J Soc Cardiac Angiography Interv. 2015;86:664–77.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Payam Dehghani declares no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Coronary Artery Disease
Rights and permissions
About this article
Cite this article
Dehghani, P. Bioresorbable Polymers and Stent Devices. Curr Treat Options Cardio Med 19, 12 (2017). https://doi.org/10.1007/s11936-017-0510-1
Published:
DOI: https://doi.org/10.1007/s11936-017-0510-1