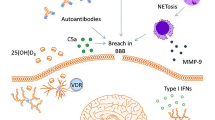
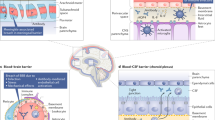
Abstract
A wide range of patients with systemic lupus erythematosus (SLE) suffer from cognitive dysfunction (CD) which severely impacts their quality of life. However, CD remains underdiagnosed and poorly understood. Here, we discuss current findings in patients and in animal models. Strong evidence suggests that CD pathogenesis involves known mechanisms of tissue injury in SLE. These mechanisms recruit brain resident cells, in particular microglia, into the pathological process. While systemic immune activation is critical to central nervous system injury, the current focus of therapy is the microglial cell and not the systemic immune perturbation. Further studies are critical to examine additional potential therapeutic targets and more specific treatments based on the cause and progress of the disease.
Similar content being viewed by others
Change history
11 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11926-021-01021-x
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Adelmann D, Saltiel E, Klinenberg J. The neuropsychiatric manifestations of SLE: an overview. Semin Arthritis Rheum. 1986;15:185–99.
Hanly J, Urowitz M, Su L, Bae SC, Gordon C, Wallace D, et al. Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Annals of the rheumatic diseases. 2010;69(3):529–35.
Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus: pathogenesis and biomarkers. Nature Reviews Neurology. 2014;10(10):579–96.
Lupus, Association AD. Lupus Foundation of America, and Lupus Research Alliance. Lupus: patient voices. Report on externallyled patient-focused drug development meeting, 2017 Sep 25, Hyattsville (MD), USA; 2018 [cited 2019 Sep 1].
Liang MH, Corzillius M, Bae SC, Lew RA, Fortin PR, Gordon C, et al. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis and rheumatism. 1999;42(4):599–608.
Zhang Z, Wang Y, Shen Z, Yang Z, Li L, Chen D, et al. The neurochemical and microstructural changes in the brain of systemic lupus erythematosus patients: A multimodal MRI study. Scientific reports. 2016;6:19026.
Al Rayes H, Tani C, Kwan A, Marzouk S, Colosimo K, Medina-Rosas J, et al. What is the prevalence of cognitive impairment in lupus and which instruments are used to measure it? A systematic review and meta-analysis. Seminars in arthritis and rheumatism: Elsevier; 2018.
Hanly J, Fisk J, Sherwood G, Jones E, Jones JV, Eastwood B. Cognitive impairment in patients with systemic lupus erythematosus. The Journal of rheumatology. 1992;19(4):562.
Hanly JG, Urowitz MB, Gordon C, Bae SC, Romero-Diaz J, Sanchez-Guerrero J, Bernatsky S, Clarke AE, Wallace DJ, Isenberg DA, Rahman A, Merrill JT, Fortin PR, Gladman DD, Bruce IN, Petri M, Ginzler EM, Dooley MA, Ramsey-Goldman R, Manzi S, Jonsen A, Alarcon GS, van Vollenhoven RF, Aranow C, Mackay M, Ruiz-Irastorza G, Lim S, Inanc M, Kalunian KC, Jacobsen S, Peschken CA, Kamen DL, Askanase A, Farewell V. Neuropsychiatric events in systemic lupus erythematosus: a longitudinal analysis of outcomes in an international inception cohort using a multistate model approach. Annals of the rheumatic diseases. 2020;79(3):356-362. Epub 2020/01/10. doi: https://doi.org/10.1136/annrheumdis-2019-216150. https://www.ncbi.nlm.nih.gov/pubmed/31915121
Joo YB, Bae SC. Assessment of clinical manifestations, disease activity and organ damage in 996 Korean patients with systemic lupus erythematosus: comparison with other Asian populations. Int J Rheum Dis. 2015;18(2):117-128. Epub 2014/12/20. https://doi.org/10.1111/1756-185X.12462. https://www.ncbi.nlm.nih.gov/pubmed/25524656
Mosca M, Costenbader KH, Johnson SR, Lorenzoni V, Sebastiani GD, Hoyer BF, Navarra S, Bonfa E, Ramsey-Goldman R, Medina-Rosas J, Piga M, Tani C, Tedeschi SK, Dorner T, Aringer M, Touma Z. Brief Report: How Do Patients With Newly Diagnosed Systemic Lupus Erythematosus Present? A Multicenter Cohort of Early Systemic Lupus Erythematosus to Inform the Development of New Classification Criteria. Arthritis Rheumatol. 2019;71(1):91-98. Epub 2018/07/24. https://doi.org/10.1002/art.40674. https://www.ncbi.nlm.nih.gov/pubmed/30035365
Pons-Estel BA, Catoggio LJ, Cardiel MH, Soriano ER, Gentiletti S, Villa AR, Abadi I, Caeiro F, Alvarellos A, Alarcon-Segovia D, Grupo Latinoamericano de Estudio del L. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among "Hispanics". Medicine. 2004;83(1):1-17. Epub 2004/01/30. https://doi.org/10.1097/01.md.0000104742.42401.e2. https://www.ncbi.nlm.nih.gov/pubmed/14747764
•• Mackay M, Vo A, Tang CC, Small M, Anderson EW, Ploran EJ, et al. Metabolic and microstructural alterations in the SLE brain correlate with cognitive impairment. JCI insight. 2019;4(1). This study found abnormalities in regional metabolic activity and interregional correlations, as well as decreased microstructural integrity and connecting tracts in WM which correlated with impaired cognitive performance and increased serum DNRAb in patients with quiescent SLE with no history of NPSLE.
Emmer BJ, van der Grond J, Steup-Beekman GM, Huizinga TW, van Buchem MA. Selective involvement of the amygdala in systemic lupus erythematosus. PLoS Med. 2006;3(12):e499. https://doi.org/10.1371/journal.pmed.0030499 PubMed PMID: 17177602; PMCID: 1702559. http://www.ncbi.nlm.nih.gov/pubmed/17177602.
Hachulla E, Michon-Pasturel U, Leys D, Pruvo JP, Queyrel V, Masy E, Arvieux J, Caron C, Brevet-Coupe F, Hatron PY, Devulder B. Cerebral magnetic resonance imaging in patients with or without antiphospholipid antibodies. Lupus. 1998;7(2):124-131. Epub 1998/04/16. https://doi.org/10.1191/096120398678919868. https://www.ncbi.nlm.nih.gov/pubmed/9541097
Kaichi Y, Kakeda S, Moriya J, Ohnari N, Saito K, Tanaka Y, Tatsugami F, Date S, Awai K, Korogi Y. Brain MR findings in patients with systemic lupus erythematosus with and without antiphospholipid antibody syndrome. AJNR Am J Neuroradiol. 2014;35(1):100-105. Epub 2013/07/28. https://doi.org/10.3174/ajnr.A3645. https://www.ncbi.nlm.nih.gov/pubmed/23886740
Valdes-Ferrer SI, Vega F, Cantu-Brito C, Ceballos-Ceballos J, Estanol B, Garcia-Ramos G, Cabral AR. Cerebral changes in SLE with or without antiphospholipid syndrome. a case-control MRI study. J Neuroimaging. 2008;18(1):62-65. Epub 2008/01/15. https://doi.org/10.1111/j.1552-6569.2007.00183.x. https://www.ncbi.nlm.nih.gov/pubmed/18190498
Zimmermann N, Correa DG, Kubo TA, Netto TM, Pereira DB, Fonseca RP, Gasparetto EL. Global Cognitive Impairment in Systemic Lupus Erythematosus Patients: A Structural MRI Study. Clin Neuroradiol. 2017;27(1):23-29. Epub 2015/05/15. https://doi.org/10.1007/s00062-015-0397-8. https://www.ncbi.nlm.nih.gov/pubmed/25967601
Sibbitt WL Jr, Brooks WM, Kornfeld M, Hart BL, Bankhurst AD, Roldan CA. Magnetic resonance imaging and brain histopathology in neuropsychiatric systemic lupus erythematosus. Seminars in arthritis and rheumatism. 2010;40(1):32–52. https://doi.org/10.1016/j.semarthrit.2009.08.005 PubMed PMID: 19880162; PMCID: 3586567. http://www.ncbi.nlm.nih.gov/pubmed/19880162.
Sibbitt WL Jr, Schmidt PJ, Hart BL, Brooks WM. Fluid Attenuated Inversion Recovery (FLAIR) imaging in neuropsychiatric systemic lupus erythematosus. The Journal of rheumatology. 2003;30(9):1983–9 http://www.ncbi.nlm.nih.gov/pubmed/12966602.
Sibbitt WL Jr, Sibbitt RR, Brooks WM. Neuroimaging in neuropsychiatric systemic lupus erythematosus. Arthritis and rheumatism. 1999;42(10):2026–38. https://doi.org/10.1002/1529-0131(199910)42:10<2026::AID-ANR2>3.0.CO;2-Jhttp://www.ncbi.nlm.nih.gov/pubmed/10524673.
Sibbitt WL Jr, Sibbitt RR, Griffey RH, Eckel C, Bankhurst AD. Magnetic resonance and computed tomographic imaging in the evaluation of acute neuropsychiatric disease in systemic lupus erythematosus. Annals of the rheumatic diseases. 1989;48(12):1014–22 PubMed PMID: 2619353; PMCID: 1003941. http://www.ncbi.nlm.nih.gov/pubmed/2619353.
Johnson RT, Richardson EP. The neurological manifestations of systemic lupus erythematosus. Medicine. 1968;47(4):337–69 http://www.ncbi.nlm.nih.gov/pubmed/5212395.
Ellis SG, Verity MA. Central nervous system involvement in systemic lupus erythematosus: a review of neuropathologic findings in 57 cases, 1955--1977. Seminars in arthritis and rheumatism. 1979;8(3):212–21 http://www.ncbi.nlm.nih.gov/pubmed/424765.
Hanly JG, Walsh NM, Sangalang V. Brain pathology in systemic lupus erythematosus. The Journal of rheumatology. 1992;19(5):732–41 http://www.ncbi.nlm.nih.gov/pubmed/1613703.
Tani C, Palagini L, Moraes-Fontes MF, Carli L, Mauri M, Bombardieri S, et al. Neuropsychiatric questionnaires in systemic lupus erythematosus. Clinical and experimental rheumatology. 2014;32(5 Suppl 85):S-59-64 Epub 2014/11/05. https://www.ncbi.nlm.nih.gov/pubmed/25365091.
Kozora E, Ellison MC, West S. Depression, fatigue, and pain in systemic lupus erythematosus (SLE): relationship to the American College of Rheumatology SLE neuropsychological battery. Arthritis Care & Research: Official Journal of the American College of Rheumatology. 2006;55(4):628–35.
Wyckoff P, Miller L, Tucker L, Schaller J. Neuropsychological assessment of children and adolescents with systemic lupus erythematosus. Lupus. 1995;4(3):217–20.
Appenzeller S, Rondina JM, Li LM, Costallat LT, Cendes F. Cerebral and corpus callosum atrophy in systemic lupus erythematosus. Arthritis & Rheumatism. 2005;52(9):2783–9.
Appenzeller S, Bonilha L, Rio PA, Li LM, Costallat LTL, Cendes F. Longitudinal analysis of gray and white matter loss in patients with systemic lupus erythematosus. Neuroimage. 2007;34(2):694–701.
Appenzeller S, Carnevalle AD, Li LM, Costallat LT, Cendes F. Hippocampal atrophy in systemic lupus erythematosus. Annals of the rheumatic diseases. 2006;65(12):1585–9.
Toyota T, Akamatsu N, Tanaka A, Shouzaki T, Tsuji S, Saito K, Tanaka Y. Mesial temporal lobe epilepsy as a neuropsychiatric syndrome of systemic lupus erythematosus. Epilepsia. 2013;54(3):e33-e36. Epub 2012/11/07. https://doi.org/10.1111/epi.12012. https://www.ncbi.nlm.nih.gov/pubmed/23126460
Liu S, Cheng Y, Zhao Y, Lai A, Lv Z, Xie Z, Upreti B, Wang X, Xu X, Luo C, Yu H, Shan B, Xu L, Xu J. Hippocampal Atrophy in Systemic Lupus Erythematosus Patients without Major Neuropsychiatric Manifestations. J Immunol Res. 2020;2020:2943848. Epub 2020/07/07. https://doi.org/10.1155/2020/2943848. PubMed PMID: 32626787; PMCID: PMC7306071. https://www.ncbi.nlm.nih.gov/pubmed/32626787
• Lauvsnes MB, Beyer MK, Kvaloy JT, Greve OJ, Appenzeller S, Kvivik I, et al. Association of hippocampal atrophy with cerebrospinal fluid antibodies against the NR2 subtype of the N-methyl-D-aspartate receptor in patients with systemic lupus erythematosus and patients with primary Sjogren's syndrome. Arthritis Rheumatol. 2014;66(12):3387–94. https://doi.org/10.1002/art.38852https://www.ncbi.nlm.nih.gov/pubmed/25156222. This study found decreased hippocampal gray matter volume in SLE patients with anti-NR2 antibodies in CSF.
West SG, Emlen W, Wener MH, Kotzin BL. Neuropsychiatric lupus erythematosus: a 10-year prospective study on the value of diagnostic tests. The American journal of medicine. 1995;99(2):153–63.
Yoshio T, Hirata D, Onda K, Nara H, Minota S. Antiribosomal P protein antibodies in cerebrospinal fluid are associated with neuropsychiatric systemic lupus erythematosus. The Journal of Rheumatology. 2005;32(1):34–9.
Fragoso-Loyo H, Cabiedes J, Orozco-Narváez A, Dávila-Maldonado L, Atisha-Fregoso Y, Diamond B, et al. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PloS one. 2008;3(10):e3347.
Ho RC, Thiaghu C, Ong H, Lu Y, Ho CS, Tam WW, et al. A meta-analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus. Autoimmunity reviews. 2016;15(2):124–38.
Chi JM, Mackay M, Hoang A, Cheng K, Aranow C, Ivanidze J, Volpe B, Diamond B, Sanelli PC. Alterations in Blood-Brain Barrier Permeability in Patients with Systemic Lupus Erythematosus. AJNR Am J Neuroradiol. 2019;40(3):470-477. Epub 2019/02/23. https://doi.org/10.3174/ajnr.A5990. PubMed PMID: 30792254; PMCID: PMC6483727. https://www.ncbi.nlm.nih.gov/pubmed/30792254
Ivanidze J, Mackay M, Hoang A, Chi JM, Cheng K, Aranow C, Volpe B, Diamond B, Sanelli PC. Dynamic contrast-enhanced MRI reveals unique blood-brain barrier permeability characteristics in the hippocampus in the normal brain. AJNR Am J Neuroradiol. 2019;40(3):408-411. Epub 2019/02/09. https://doi.org/10.3174/ajnr.A5962. PubMed PMID: 30733256; PMCID: PMC7028673. https://www.ncbi.nlm.nih.gov/pubmed/30733256
Fragoso-Loyo H, Cabiedes J, Richaud-Patin Y, Orozco-Narvaez A, Diamond B, Llorente L, et al. Inflammatory profile in the cerebrospinal fluid of patients with central neuropsychiatric lupus, with and without associated factors. Rheumatology. 2009;48(12):1615–6. https://doi.org/10.1093/rheumatology/kep297 PubMed PMID: 19755508; PMCID: PMC4516016. https://www.ncbi.nlm.nih.gov/pubmed/19755508.
Kwieciński J, Kłak M, Trysberg E, Blennow K, Tarkowski A, Jin T. Relationship between elevated cerebrospinal fluid levels of plasminogen activator inhibitor 1 and neuronal destruction in patients with neuropsychiatric systemic lupus erythematosus. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 2009;60(7):2094–101.
Shiozawa S, Kuroki Y, Kim M, Hirohata S, Ogino T. Interferon-alpha in lupus psychosis. Arthritis and rheumatism. 1992;35(4):417-422. Epub 1992/04/01. https://doi.org/10.1002/art.1780350410. https://www.ncbi.nlm.nih.gov/pubmed/1373622
Aw E, Zhang Y, Carroll M. Microglial responses to peripheral type 1 interferon. J Neuroinflammation. 2020;17(1):340. Epub 2020/11/14. https://doi.org/10.1186/s12974-020-02003-z. PubMed PMID: 33183319; PMCID: PMC7659169. https://www.ncbi.nlm.nih.gov/pubmed/33183319
Bialas AR, Presumey J, Das A, van der Poel CE, Lapchak PH, Mesin L, et al. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature. 2017;546(7659):539–43.
Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P, et al. Anifrolumab, an anti–interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis & rheumatology. 2017;69(2):376–86.
Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences. 2009;106(6):1942–7.
Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain. 2010;133(5):1352–67.
Vogelgesang S, Heyes M, West S, Salazar A, Sfikakis P, Lipnick R, et al. Quinolinic acid in patients with systemic lupus erythematosus and neuropsychiatric manifestations. The Journal of Rheumatology. 1996;23(5):850–5.
Stojanovich L, Smiljanich-Miljkovich D, Omdal R, Sakic B. Neuropsychiatric lupus and association with cerebrospinal fluid immunoglobulins: a pilot study. The Israel Medical Association journal: IMAJ. 2009;11(6):359.
Trysberg E, Nylen K, Rosengren LE, Tarkowski A. Neuronal and astrocytic damage in systemic lupus erythematosus patients with central nervous system involvement. Arthritis & Rheumatism. 2003;48(10):2881–7.
Boulamery A, Desplat-Jego S. Regulation of neuroinflammation: what role for the tumor necrosis factor-like weak inducer of apoptosis/Fn14 pathway? Frontiers in immunology. 2017;8:1534. Epub 2017/12/05. https://doi.org/10.3389/fimmu.2017.01534. PubMed PMID: 29201025; PMCID: PMC5696327. https://www.ncbi.nlm.nih.gov/pubmed/29201025
Fragoso-Loyo H, Richaud-Patin Y, Orozco-Narvaez A, Davila-Maldonado L, Atisha-Fregoso Y, Llorente L, et al. Interleukin-6 and chemokines in the neuropsychiatric manifestations of systemic lupus erythematosus. Arthritis and rheumatism. 2007;56(4):1242–50. https://doi.org/10.1002/art.22451http://www.ncbi.nlm.nih.gov/pubmed/17393453.
Wen J, Xia Y, Stock A, Michaelson JS, Burkly LC, Gulinello M, et al. Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway. Journal of autoimmunity. 2013;43:44–54.
Shimizu F, Schaller KL, Owens GP, Cotleur AC, Kellner D, Takeshita Y, Obermeier B, Kryzer TJ, Sano Y, Kanda T, Lennon VA, Ransohoff RM, Bennett JL. Glucose-regulated protein 78 autoantibody associates with blood-brain barrier disruption in neuromyelitis optica. Sci Transl Med. 2017;9(397). Epub 2017/07/07. https://doi.org/10.1126/scitranslmed.aai9111. PubMed PMID: 28679661; PMCID: PMC5585784. https://www.ncbi.nlm.nih.gov/pubmed/28679661
Zavada J, Nytrova P, Wandinger KP, Jarius S, Svobodova R, Probst C, Peterova V, Tegzova D, Pavelka K, Vencovsky J. Seroprevalence and specificity of NMO-IgG (anti-aquaporin 4 antibodies) in patients with neuropsychiatric systemic lupus erythematosus. Rheumatology international. 2013;33(1):259-263. Epub 2011/11/01. https://doi.org/10.1007/s00296-011-2176-4. https://www.ncbi.nlm.nih.gov/pubmed/22038193
Matsueda Y, Arinuma Y, Nagai T, Hirohata S. Elevation of serum anti-glucose-regulated protein 78 antibodies in neuropsychiatric systemic lupus erythematosus. Lupus Sci Med. 2018;5(1):e000281. Epub 2018/11/07. https://doi.org/10.1136/lupus-2018-000281. PubMed PMID: 30397496; PMCID: PMC6203046. https://www.ncbi.nlm.nih.gov/pubmed/30397496
Migliorini P, Baldini C, Rocchi V, Bombardieri S. Anti-Sm and anti-RNP antibodies. Autoimmunity. 2005;38(1):47-54. Epub 2005/04/05. https://doi.org/10.1080/08916930400022715. https://www.ncbi.nlm.nih.gov/pubmed/15804705
Yoshio T, Okamoto H, Hirohata S, Minota S. IgG anti-NR2 glutamate receptor autoantibodies from patients with systemic lupus erythematosus activate endothelial cells. Arthritis and rheumatism. 2013;65(2):457-463. Epub 2012/10/12. https://doi.org/10.1002/art.37745. https://www.ncbi.nlm.nih.gov/pubmed/23055186
Muslimov IA, Iacoangeli A, Eom T, Ruiz A, Lee M, Stephenson S, Ginzler EM, Tiedge H. Neuronal BC RNA Transport impairments caused by systemic lupus erythematosus autoantibodies. J Neurosci. 2019;39(39):7759-77. Epub 2019/08/14. https://doi.org/10.1523/JNEUROSCI.1657-18.2019. PubMed PMID: 31405929; PMCID: PMC6764197. https://www.ncbi.nlm.nih.gov/pubmed/31405929
Briz V, Restivo L, Pasciuto E, Juczewski K, Mercaldo V, Lo AC, Baatsen P, Gounko NV, Borreca A, Girardi T, Luca R, Nys J, Poorthuis RB, Mansvelder HD, Fisone G, Ammassari-Teule M, Arckens L, Krieger P, Meredith R, Bagni C. The non-coding RNA BC1 regulates experience-dependent structural plasticity and learning. Nat Commun. 2017;8(1):293. Epub 2017/08/19. https://doi.org/10.1038/s41467-017-00311-2. PubMed PMID: 28819097; PMCID: PMC5561022. https://www.ncbi.nlm.nih.gov/pubmed/28819097
Karnopp TE, Chapacais GF, Freitas EC, Monticielo OA. Lupus animal models and neuropsychiatric implications. Clinical rheumatology. 2020. Epub 2020/11/07. https://doi.org/10.1007/s10067-020-05493-7. https://www.ncbi.nlm.nih.gov/pubmed/33155159
Li W, Titov AA, Morel L. An update on lupus animal models. Curr Opin Rheumatol. 2017;29(5):434-41. Epub 2017/05/26. https://doi.org/10.1097/BOR.0000000000000412. PubMed PMID: 28537986; PMCID: PMC5815391. https://www.ncbi.nlm.nih.gov/pubmed/28537986
Mike EV, Makinde HM, Gulinello M, Vanarsa K, Herlitz L, Gadhvi G, Winter DR, Mohan C, Hanly JG, Mok CC, Cuda CM, Putterman C. Lipocalin-2 is a pathogenic determinant and biomarker of neuropsychiatric lupus. Journal of autoimmunity. 2019;96:59-73. Epub 2018/09/04. https://doi.org/10.1016/j.jaut.2018.08.005. PubMed PMID: 30174216; PMCID: PMC6310639. https://www.ncbi.nlm.nih.gov/pubmed/30174216
Chu JL, Drappa J, Parnassa A, Elkon KB. The defect in Fas mRNA expression in MRL/lpr mice is associated with insertion of the retrotransposon, ETn. The Journal of experimental medicine. 1993;178(2):723-30. Epub 1993/08/01. https://doi.org/10.1084/jem.178.2.723. PubMed PMID: 7688033; PMCID: PMC2191101. https://www.ncbi.nlm.nih.gov/pubmed/7688033
Dixon FJ, Andrews BS, Eisenberg RA, McConahey PJ, Theofilopoulos AN, Wilson CB. Etiology and pathogenesis of a spontaneous lupus-like syndrome in mice. Arthritis and rheumatism. 1978;21(5 Suppl):S64-S67. Epub 1978/06/01. https://doi.org/10.1002/art.1780210909. https://www.ncbi.nlm.nih.gov/pubmed/307393
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356(6367):314-317. Epub 1992/03/26. https://doi.org/10.1038/356314a0. https://www.ncbi.nlm.nih.gov/pubmed/1372394
Watson ML, Rao JK, Gilkeson GS, Ruiz P, Eicher EM, Pisetsky DS, Matsuzawa A, Rochelle JM, Seldin MF. Genetic analysis of MRL-lpr mice: relationship of the Fas apoptosis gene to disease manifestations and renal disease-modifying loci. The Journal of experimental medicine. 1992;176(6):1645-56. Epub 1992/12/01. https://doi.org/10.1084/jem.176.6.1645. PubMed PMID: 1460423; PMCID: PMC2119463. https://www.ncbi.nlm.nih.gov/pubmed/1460423
Gao HX, Campbell SR, Cui MH, Zong P, Hee-Hwang J, Gulinello M, Putterman C. Depression is an early disease manifestation in lupus-prone MRL/lpr mice. J Neuroimmunol. 2009;207(1-2):45-56. https://doi.org/10.1016/j.jneuroim.2008.11.009. PubMed PMID: 19121871; PMCID: 2675630. http://www.ncbi.nlm.nih.gov/pubmed/19121871
Gao H-X, Sanders E, Tieng AT, Putterman C. Sex and autoantibody titers determine the development of neuropsychiatric manifestations in lupus-prone mice. Journal of neuroimmunology. 2010;229(1-2):112–22.
Šakić B, Szechtman H, Keffer M, Talangbayan H, Stead R, Denburg JA. A behavioral profile of autoimmune lupus-prone MRL mice. Brain, behavior, and immunity. 1992;6(3):265–85.
Šakić B, Szechtman H, Talangbayan H, Denburg SD, Carbotte RM, Denburg JA. Disturbed emotionality in autoimmune MRL-lpr mice. Physiology & behavior. 1994;56(3):609–17.
Vogelweid CM, Wright DC, Johnson JC, Hewett JE, Walker SE. Evaluation of memory, learning ability, and clinical neurologic function in pathogen-free mice with systemic lupus erythematosus. Arthritis and rheumatism. 1994;37(6):889-897. Epub 1994/06/01. https://doi.org/10.1002/art.1780370617. https://www.ncbi.nlm.nih.gov/pubmed/8003061
Sakic B, Denburg JA, Denburg SD, Szechtman H. Blunted sensitivity to sucrose in autoimmune MRL-lpr mice: a curve-shift study. Brain Res Bull. 1996;41(5):305-311. Epub 1996/01/01. https://doi.org/10.1016/s0361-9230(96)00190-6. https://www.ncbi.nlm.nih.gov/pubmed/8924042
Nielsen DM, Crnic LS. Elevated plus maze behavior, auditory startle response, and shock sensitivity in predisease and in early stage autoimmune disease MRL/lpr mice. Brain Behav Immun. 2002;16(1):46-61. Epub 2002/02/16. https://doi.org/10.1006/brbi.2000.0610. https://www.ncbi.nlm.nih.gov/pubmed/11846440
Ballok DA, Woulfe J, Sur M, Cyr M, Sakic B. Hippocampal damage in mouse and human forms of systemic autoimmune disease. Hippocampus. 2004;14(5):649-61. Epub 2004/08/11. https://doi.org/10.1002/hipo.10205. PubMed PMID: 15301441; PMCID: PMC1764443. https://www.ncbi.nlm.nih.gov/pubmed/15301441
Ma X, Foster J, Sakic B. Distribution and prevalence of leukocyte phenotypes in brains of lupus-prone mice. J Neuroimmunol. 2006;179(1-2):26-36. Epub 2006/08/15. https://doi.org/10.1016/j.jneuroim.2006.06.023. https://www.ncbi.nlm.nih.gov/pubmed/16904195
Mike EV, Makinde HM, Der E, Stock A, Gulinello M, Gadhvi GT, Winter DR, Cuda CM, Putterman C. Neuropsychiatric systemic lupus erythematosus is dependent on sphingosine-1-phosphate signaling. Frontiers in immunology. 2018;9:2189. Epub 2018/10/16. https://doi.org/10.3389/fimmu.2018.02189. PubMed PMID: 30319641; PMCID: PMC6168636. https://www.ncbi.nlm.nih.gov/pubmed/30319641
Sakic B. A novel experimental approach in treating central nervous system lupus: kudos and kicks. Arthritis and rheumatism. 2009;60(12):3531–3. https://doi.org/10.1002/art.25016http://www.ncbi.nlm.nih.gov/pubmed/19950267.
Šakić B, Szechtman H, Denburg JA, Gorny G, Kolb B, Whishaw IQ. Progressive atrophy of pyramidal neuron dendrites in autoimmune MRL-lpr mice. Journal of neuroimmunology. 1998;87(1-2):162–70.
Maric D, Millward JM, Ballok DA, Szechtman H, Denburg JA, Barker JL, Sakic B. Neurotoxic properties of cerebrospinal fluid from behaviorally impaired autoimmune mice. Brain research. 2001;920(1-2):183-193. Epub 2001/11/22. https://doi.org/10.1016/s0006-8993(01)03060-8. https://www.ncbi.nlm.nih.gov/pubmed/11716824
Sakic B. The MRL model: an invaluable tool in studies of autoimmunity-brain interactions. Methods in molecular biology. 2012;934:277-299. Epub 2012/08/31. https://doi.org/10.1007/978-1-62703-071-7_14. https://www.ncbi.nlm.nih.gov/pubmed/22933151
Sidor MM, Sakic B, Malinowski PM, Ballok DA, Oleschuk CJ, Macri J. Elevated immunoglobulin levels in the cerebrospinal fluid from lupus-prone mice. J Neuroimmunol. 2005;165(1-2):104-13. Epub 2005/06/24. https://doi.org/10.1016/j.jneuroim.2005.04.022. PubMed PMID: 15972238; PMCID: PMC1635784. https://www.ncbi.nlm.nih.gov/pubmed/15972238
Stanojcic M, Loheswaran G, Xu L, Hoffman SA, Sakic B. Intrathecal antibodies and brain damage in autoimmune MRL mice. Brain Behav Immun. 2010;24(2):289-297. Epub 2009/10/27. https://doi.org/10.1016/j.bbi.2009.10.009. https://www.ncbi.nlm.nih.gov/pubmed/19853033
Li P, Lin W, Zheng X. IL-33 neutralization suppresses lupus disease in lupus-prone mice. Inflammation. 2014;37(3):824-832. Epub 2014/01/09. doi: https://doi.org/10.1007/s10753-013-9802-0. https://www.ncbi.nlm.nih.gov/pubmed/24398614
Tang B, Matsuda T, Akira S, Nagata N, Ikehara S, Hirano T, Kishimoto T. Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol. 1991;3(3):273-278. Epub 1991/03/01. https://doi.org/10.1093/intimm/3.3.273. https://www.ncbi.nlm.nih.gov/pubmed/2049341
Tomita M, Khan RL, Blehm BH, Santoro TJ. The potential pathogenetic link between peripheral immune activation and the central innate immune response in neuropsychiatric systemic lupus erythematosus. Med Hypotheses. 2004;62(3):325-335. Epub 2004/02/21. https://doi.org/10.1016/j.mehy.2003.10.009. https://www.ncbi.nlm.nih.gov/pubmed/14975498
Tsai CY, Wu TH, Huang SF, Sun KH, Hsieh SC, Han SH, Yu HS, Yu CL. Abnormal splenic and thymic IL-4 and TNF-alpha expression in MRL-lpr/lpr mice. Scand J Immunol. 1995;41(2):157-163. Epub 1995/02/01. https://doi.org/10.1111/j.1365-3083.1995.tb03548.x. https://www.ncbi.nlm.nih.gov/pubmed/7863262
Alexander JJ, Jacob A, Bao L, Macdonald RL, Quigg RJ. Complement-dependent apoptosis and inflammatory gene changes in murine lupus cerebritis. Journal of immunology. 2005;175(12):8312-8319. Epub 2005/12/13. https://doi.org/10.4049/jimmunol.175.12.8312. https://www.ncbi.nlm.nih.gov/pubmed/16339572
Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biological psychiatry. 2006;59(9):775-785. Epub 2006/02/07. https://doi.org/10.1016/j.biopsych.2005.10.013. https://www.ncbi.nlm.nih.gov/pubmed/16458261
Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153-60. Epub 2006/11/08. https://doi.org/10.1016/j.bbi.2006.09.006. PubMed PMID: 17088043; PMCID: PMC1850954. https://www.ncbi.nlm.nih.gov/pubmed/17088043
McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neuroscience and biobehavioral reviews. 2009;33(3):355-366. Epub 2008/11/11. https://doi.org/10.1016/j.neubiorev.2008.10.005. http://www.ncbi.nlm.nih.gov/pubmed/18996146
Wu TH, Lin CH. IL-6 mediated alterations on immobile behavior of rats in the forced swim test via ERK1/2 activation in specific brain regions. Behavioural brain research. 2008;193(2):183-191. Epub 2008/06/25. https://doi.org/10.1016/j.bbr.2008.05.009. https://www.ncbi.nlm.nih.gov/pubmed/18573547
Saija A, Princi P, Lanza M, Scalese M, Aramnejad E, De Sarro A. Systemic cytokine administration can affect blood-brain barrier permeability in the rat. Life Sci. 1995;56(10):775-784. Epub 1995/01/01. https://doi.org/10.1016/0024-3205(95)00008-t. https://www.ncbi.nlm.nih.gov/pubmed/7885193
Veldhuis WB, Floris S, van der Meide PH, Vos IM, de Vries HE, Dijkstra CD, Bar PR, Nicolay K. Interferon-beta prevents cytokine-induced neutrophil infiltration and attenuates blood-brain barrier disruption. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23(9):1060-1069. Epub 2003/09/16. https://doi.org/10.1097/01.WCB.0000080701.47016.24. https://www.ncbi.nlm.nih.gov/pubmed/12973022
Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine. 2007;40(1):1-16. Epub 2007/11/06. https://doi.org/10.1016/j.cyto.2007.09.007. https://www.ncbi.nlm.nih.gov/pubmed/17981048
Crampton SP, Morawski PA, Bolland S. Linking susceptibility genes and pathogenesis mechanisms using mouse models of systemic lupus erythematosus. Dis Model Mech. 2014;7(9):1033-46. Epub 2014/08/26. https://doi.org/10.1242/dmm.016451. PubMed PMID: 25147296; PMCID: PMC4142724. https://www.ncbi.nlm.nih.gov/pubmed/25147296
Rozzo SJ, Vyse TJ, David CS, Palmer E, Izui S, Kotzin BL. Analysis of MHC class II genes in the susceptibility to lupus in New Zealand mice. Journal of immunology. 1999;162(5):2623-2630. Epub 1999/03/11. https://www.ncbi.nlm.nih.gov/pubmed/10072504
Vyse TJ, Rozzo SJ, Drake CG, Appel VB, Lemeur M, Izui S, Palmer E, Kotzin BL. Contributions of Ea(z) and Eb(z) MHC genes to lupus susceptibility in New Zealand mice. Journal of immunology. 1998;160(6):2757-2766. Epub 1998/03/24. https://www.ncbi.nlm.nih.gov/pubmed/9510177
Shi D, Tian T, Yao S, Cao K, Zhu X, Zhang M, Wen S, Li L, Shi M, Zhou H. FTY720 attenuates behavioral deficits in a murine model of systemic lupus erythematosus. Brain Behav Immun. 2018;70:293-304. Epub 2018/03/20. https://doi.org/10.1016/j.bbi.2018.03.009. https://www.ncbi.nlm.nih.gov/pubmed/29548997
Drake CG, Rozzo SJ, Hirschfeld HF, Smarnworawong NP, Palmer E, Kotzin BL. Analysis of the New Zealand Black contribution to lupus-like renal disease. Multiple genes that operate in a threshold manner. Journal of immunology. 1995;154(5):2441-2447. Epub 1995/03/01. https://www.ncbi.nlm.nih.gov/pubmed/7868910
Helyer BJ, Howie JB. Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature. 1963;197:197. Epub 1963/01/12. https://doi.org/10.1038/197197a0. https://www.ncbi.nlm.nih.gov/pubmed/13953664
Wu WM, Lin BF, Su YC, Suen JL, Chiang BL. Tamoxifen decreases renal inflammation and alleviates disease severity in autoimmune NZB/W F1 mice. Scand J Immunol. 2000;52(4):393-400. Epub 2000/09/30. https://doi.org/10.1046/j.1365-3083.2000.00789.x. https://www.ncbi.nlm.nih.gov/pubmed/11013011
Kier AB. Clinical neurology and brain histopathology in NZB/NZW F1 lupus mice. J Comp Pathol. 1990;102(2):165-177. Epub 1990/02/01. https://doi.org/10.1016/s0021-9975(08)80122-3. https://www.ncbi.nlm.nih.gov/pubmed/2324339
Zhuang H, Szeto C, Han S, Yang L, Reeves WH. Animal Models of Interferon Signature Positive Lupus. Frontiers in immunology. 2015;6:291. Epub 2015/06/23. https://doi.org/10.3389/fimmu.2015.00291. PubMed PMID: 26097482; PMCID: PMC4456949. https://www.ncbi.nlm.nih.gov/pubmed/26097482
Schrott LM, Crnic LS. Anxiety behavior, exploratory behavior, and activity in NZB x NZW F1 hybrid mice: role of genotype and autoimmune disease progression. Brain Behav Immun. 1996;10(3):260-274. Epub 1996/09/01. https://doi.org/10.1006/brbi.1996.0023. https://www.ncbi.nlm.nih.gov/pubmed/8954598
Bracci-Laudiero L, Aloe L, Lundeberg T, Theodorsson E, Stenfors C. Altered levels of neuropeptides characterize the brain of lupus prone mice. Neuroscience letters. 1999;275(1):57-60. Epub 1999/11/11. https://doi.org/10.1016/s0304-3940(99)00737-5. https://www.ncbi.nlm.nih.gov/pubmed/10554984
Leung JW, Lau BW, Chan VS, Lau CS, So KF. Abnormal increase of neuronal precursor cells and exacerbated neuroinflammation in the corpus callosum in murine model of systemic lupus erythematosus. Restorative neurology and neuroscience. 2016;34(3):443-53. Epub 2016/05/11. https://doi.org/10.3233/RNN-160638. PubMed PMID: 27163251; PMCID: PMC4927870. https://www.ncbi.nlm.nih.gov/pubmed/27163251
Sherman GF, Morrison L, Rosen GD, Behan PO, Galaburda AM. Brain abnormalities in immune defective mice. Brain research. 1990;532(1-2):25-33. Epub 1990/11/05. https://doi.org/10.1016/0006-8993(90)91737-2. https://www.ncbi.nlm.nih.gov/pubmed/2282519
Rudofsky UH, Evans BD, Balaban SL, Mottironi VD, Gabrielsen AE. Differences in expression of lupus nephritis in New Zealand mixed H-2z homozygous inbred strains of mice derived from New Zealand black and New Zealand white mice. Origins and initial characterization. Lab Invest. 1993;68(4):419-426. Epub 1993/04/01. https://www.ncbi.nlm.nih.gov/pubmed/8479150
Rudofsky UH, Lawrence DA. New Zealand mixed mice: a genetic systemic lupus erythematosus model for assessing environmental effects. Environ Health Perspect. 1999;107 Suppl 5:713-21. Epub 1999/09/30. https://doi.org/10.1289/ehp.99107s5713. PubMed PMID: 10502536; PMCID: PMC1566260. https://www.ncbi.nlm.nih.gov/pubmed/10502536
Morel L. Mapping lupus susceptibility genes in the NZM2410 mouse model. Adv Immunol. 2012;115:113-139. Epub 2012/05/23. https://doi.org/10.1016/B978-0-12-394299-9.00004-7. https://www.ncbi.nlm.nih.gov/pubmed/22608257
Mohan C, Morel L, Yang P, Watanabe H, Croker B, Gilkeson G, Wakeland EK. Genetic dissection of lupus pathogenesis: a recipe for nephrophilic autoantibodies. The Journal of clinical investigation. 1999;103(12):1685-95. Epub 1999/06/22. https://doi.org/10.1172/JCI5827. PubMed PMID: 10377175; PMCID: PMC408382. https://www.ncbi.nlm.nih.gov/pubmed/10377175
Suk K. Lipocalin-2 as a therapeutic target for brain injury: An astrocentric perspective. Progress in neurobiology. 2016;144:158-172. Epub 2016/08/09. https://doi.org/10.1016/j.pneurobio.2016.08.001. https://www.ncbi.nlm.nih.gov/pubmed/27498195
Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, Li F, Xu Z, Bowser R, Xia XG, Zhou H. Reactive astrocytes secrete lcn2 to promote neuron death. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(10):4069-74. Epub 2013/02/23. https://doi.org/10.1073/pnas.1218497110. PubMed PMID: 23431168; PMCID: PMC3593910. https://www.ncbi.nlm.nih.gov/pubmed/23431168
Ip JP, Nocon AL, Hofer MJ, Lim SL, Muller M, Campbell IL. Lipocalin 2 in the central nervous system host response to systemic lipopolysaccharide administration. J Neuroinflammation. 2011;8:124. Epub 2011/09/29. https://doi.org/10.1186/1742-2094-8-124. PubMed PMID: 21943033; PMCID: PMC3192694. https://www.ncbi.nlm.nih.gov/pubmed/21943033
Jin M, Kim JH, Jang E, Lee YM, Soo Han H, Woo DK, Park DH, Kook H, Suk K. Lipocalin-2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34(8):1306-14. Epub 2014/05/02. https://doi.org/10.1038/jcbfm.2014.83. PubMed PMID: 24780901; PMCID: PMC4126090. https://www.ncbi.nlm.nih.gov/pubmed/24780901
Lee S, Kim JH, Kim JH, Seo JW, Han HS, Lee WH, Mori K, Nakao K, Barasch J, Suk K. Lipocalin-2 Is a chemokine inducer in the central nervous system: role of chemokine ligand 10 (CXCL10) in lipocalin-2-induced cell migration. J Biol Chem. 2011;286(51):43855-70. Epub 2011/10/28. https://doi.org/10.1074/jbc.M111.299248. PubMed PMID: 22030398; PMCID: PMC3243551. https://www.ncbi.nlm.nih.gov/pubmed/22030398
Lee S, Lee J, Kim S, Park JY, Lee WH, Mori K, Kim SH, Kim IK, Suk K. A dual role of lipocalin 2 in the apoptosis and deramification of activated microglia. Journal of immunology. 2007;179(5):3231-3241. Epub 2007/08/22. https://doi.org/10.4049/jimmunol.179.5.3231. https://www.ncbi.nlm.nih.gov/pubmed/17709539
Marques F, Rodrigues AJ, Sousa JC, Coppola G, Geschwind DH, Sousa N, Correia-Neves M, Palha JA. Lipocalin 2 is a choroid plexus acute-phase protein. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28(3):450-455. Epub 2007/09/27. https://doi.org/10.1038/sj.jcbfm.9600557. https://www.ncbi.nlm.nih.gov/pubmed/17895910
Mucha M, Skrzypiec AE, Schiavon E, Attwood BK, Kucerova E, Pawlak R. Lipocalin-2 controls neuronal excitability and anxiety by regulating dendritic spine formation and maturation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(45):18436-41. Epub 2011/10/05. https://doi.org/10.1073/pnas.1107936108. PubMed PMID: 21969573; PMCID: PMC3215032. https://www.ncbi.nlm.nih.gov/pubmed/21969573
Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27(5):801-10. Epub 2007/11/13. https://doi.org/10.1016/j.immuni.2007.09.009. PubMed PMID: 17997333; PMCID: PMC2706502. https://www.ncbi.nlm.nih.gov/pubmed/17997333
Murphy ED, Roths JB. A Y chromosome associated factor in strain BXSB producing accelerated autoimmunity and lymphoproliferation. Arthritis and rheumatism. 1979;22(11):1188-1194. Epub 1979/11/01. https://doi.org/10.1002/art.1780221105. https://www.ncbi.nlm.nih.gov/pubmed/315777
Cuda CM, Misharin AV, Gierut AK, Saber R, Haines GK, 3rd, Hutcheson J, Hedrick SM, Mohan C, Budinger GS, Stehlik C, Perlman H. Caspase-8 acts as a molecular rheostat to limit RIPK1- and MyD88-mediated dendritic cell activation. Journal of immunology. 2014;192(12):5548-60. Epub 2014/05/09. https://doi.org/10.4049/jimmunol.1400122. PubMed PMID: 24808358; PMCID: PMC4074511. https://www.ncbi.nlm.nih.gov/pubmed/24808358
Makinde HM, Winter DR, Procissi D, Mike EV, Stock AD, Kando MJ, Gadhvi GT, Droho S, Bloomfield CL, Dominguez ST, Mayr MG, Lavine JA, Putterman C, Cuda CM. A novel microglia-specific transcriptional signature correlates with behavioral deficits in neuropsychiatric lupus. Frontiers in immunology. 2020;11:230. Epub 2020/03/17. https://doi.org/10.3389/fimmu.2020.00230. PubMed PMID: 32174913; PMCID: PMC7055359. https://www.ncbi.nlm.nih.gov/pubmed/32174913
Deczkowska A, Keren-Shaul H, Weiner A, Colonna M, Schwartz M, Amit I. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell. 2018;173(5):1073-1081. Epub 2018/05/19. https://doi.org/10.1016/j.cell.2018.05.003. https://www.ncbi.nlm.nih.gov/pubmed/29775591
McDonald G, Medina CO, Pilichowska M, Kearney JF, Shinkura R, Selsing E, Wortis HH, Honjo T, Imanishi-Kari T. Accelerated systemic autoimmunity in the absence of somatic hypermutation in 564Igi: a mouse model of systemic lupus with knocked-in heavy and light chain genes. Frontiers in immunology. 2017;8:1094. Epub 2017/09/29. https://doi.org/10.3389/fimmu.2017.01094. PubMed PMID: 28955333; PMCID: PMC5601273. https://www.ncbi.nlm.nih.gov/pubmed/28955333
Berland R, Fernandez L, Kari E, Han JH, Lomakin I, Akira S, Wortis HH, Kearney JF, Ucci AA, Imanishi-Kari T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25(3):429-440. Epub 2006/09/16. https://doi.org/10.1016/j.immuni.2006.07.014. https://www.ncbi.nlm.nih.gov/pubmed/16973388
Han JH, Umiker BR, Kazimirova AA, Fray M, Korgaonkar P, Selsing E, Imanishi-Kari T. Expression of an anti-RNA autoantibody in a mouse model of SLE increases neutrophil and monocyte numbers as well as IFN-I expression. European journal of immunology. 2014;44(1):215-26. Epub 2013/10/10. https://doi.org/10.1002/eji.201343714. PubMed PMID: 24105635; PMCID: PMC3947137. https://www.ncbi.nlm.nih.gov/pubmed/24105635
Freitas EC, de Oliveira MS, Monticielo OA. Pristane-induced lupus: considerations on this experimental model. Clinical rheumatology. 2017;36(11):2403-2414. Epub 2017/09/08. https://doi.org/10.1007/s10067-017-3811-6. https://www.ncbi.nlm.nih.gov/pubmed/28879482
Reeves WH, Lee PY, Weinstein JS, Satoh M, Lu L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends in immunology. 2009;30(9):455-464. Epub 2009/08/25. https://doi.org/10.1016/j.it.2009.06.003. PubMed PMID: 19699150; PMCID: PMC2746238. https://www.ncbi.nlm.nih.gov/pubmed/19699150
Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. The Journal of experimental medicine. 1998;188(5):985-90. Epub 1998/09/09. https://doi.org/10.1084/jem.188.5.985. PubMed PMID: 9730900; PMCID: PMC2213386. https://www.ncbi.nlm.nih.gov/pubmed/9730900
Shaheen VM, Satoh M, Richards HB, Yoshida H, Shaw M, Jennette JC, Reeves WH. Immunopathogenesis of environmentally induced lupus in mice. Environ Health Perspect. 1999;107 Suppl 5:723-7. Epub 1999/09/30. https://doi.org/10.1289/ehp.99107s5723. PubMed PMID: 10502537; PMCID: PMC1566261. https://www.ncbi.nlm.nih.gov/pubmed/10502537
Luciano-Jaramillo J, Sandoval-Garcia F, Vazquez-Del Mercado M, Gutierrez-Mercado YK, Navarro-Hernandez RE, Martinez-Garcia EA, Pizano-Martinez O, Corona-Meraz FI, Banuelos-Pineda J, Floresvillar-Mosqueda JF, Martin-Marquez BT. Downregulation of hippocampal NR2A/2B subunits related to cognitive impairment in a pristane-induced lupus BALB/c mice. PLoS One. 2019;14(9):e0217190. Epub 2019/09/10. https://doi.org/10.1371/journal.pone.0217190. PubMed PMID: 31498792; PMCID: PMC6733477. https://www.ncbi.nlm.nih.gov/pubmed/31498792
Faust TW, Chang EH, Kowal C, Berlin R, Gazaryan IG, Bertini E, et al. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proceedings of the National Academy of Sciences. 2010;107(43):18569–74.
Chan K, Nestor J, Huerta TS, Certain N, Moody G, Kowal C, et al. Lupus auto-antibodies act as positive allosteric modulators at NMDA receptors and induce spatial memory deficits. bioRxiv. 2019:791715.
Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramon E, Buonaiuto V, Jacobsen S, Zeher MM, Tarr T, Tincani A, Taglietti M, Theodossiades G, Nomikou E, Galeazzi M, Bellisai F, Meroni PL, Derksen RH, de Groot PG, Baleva M, Mosca M, Bombardieri S, Houssiau F, Gris JC, Quere I, Hachulla E, Vasconcelos C, Fernandez-Nebro A, Haro M, Amoura Z, Miyara M, Tektonidou M, Espinosa G, Bertolaccini ML, Khamashta MA, Euro-Phospholipid Project G. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Annals of the rheumatic diseases. 2015;74(6):1011-1018. Epub 2014/01/28. https://doi.org/10.1136/annrheumdis-2013-204838. https://www.ncbi.nlm.nih.gov/pubmed/24464962
Yelnik CM, Kozora E, Appenzeller S. Non-stroke central neurologic manifestations in antiphospholipid syndrome. Current rheumatology reports. 2016;18(2):11. Epub 2016/03/01. https://doi.org/10.1007/s11926-016-0568-x. https://www.ncbi.nlm.nih.gov/pubmed/26923254
Yelnik CM, Kozora E, Appenzeller S. Cognitive disorders and antiphospholipid antibodies. Autoimmunity reviews. 2016;15(12):1193-1198. Epub 2016/09/19. https://doi.org/10.1016/j.autrev.2016.09.002. https://www.ncbi.nlm.nih.gov/pubmed/27639839
Frauenknecht K, Katzav A, Weiss Lavi R, Sabag A, Otten S, Chapman J, Sommer CJ. Mice with experimental antiphospholipid syndrome display hippocampal dysfunction and a reduction of dendritic complexity in hippocampal CA1 neurones. Neuropathology and applied neurobiology. 2015;41(5):657-671. Epub 2014/09/10. https://doi.org/10.1111/nan.12180. https://www.ncbi.nlm.nih.gov/pubmed/25201289
Gharavi EE, Chaimovich H, Cucurull E, Celli CM, Tang H, Wilson WA, Gharavi AE. Induction of antiphospholipid antibodies by immunization with synthetic viral and bacterial peptides. Lupus. 1999;8(6):449-455. Epub 1999/09/14. https://doi.org/10.1177/096120339900800607. https://www.ncbi.nlm.nih.gov/pubmed/10483013
Katzav A, Ben-Ziv T, Blank M, Pick CG, Shoenfeld Y, Chapman J. Antibody-specific behavioral effects: intracerebroventricular injection of antiphospholipid antibodies induces hyperactive behavior while anti-ribosomal-P antibodies induces depression and smell deficits in mice. J Neuroimmunol. 2014;272(1-2):10-15. Epub 2014/05/20. https://doi.org/10.1016/j.jneuroim.2014.04.003. https://www.ncbi.nlm.nih.gov/pubmed/24837568
Katzav A, Pick CG, Korczyn AD, Oest E, Blank M, Shoenfeld Y, Chapman J. Hyperactivity in a mouse model of the antiphospholipid syndrome. Lupus. 2001;10(7):496-499. Epub 2001/08/02. doi: https://doi.org/10.1191/096120301678416060. https://www.ncbi.nlm.nih.gov/pubmed/11480848
Frauenknecht K, Katzav A, Grimm C, Chapman J, Sommer CJ. Neurological impairment in experimental antiphospholipid syndrome is associated with increased ligand binding to hippocampal and cortical serotonergic 5-HT1A receptors. Immunobiology. 2013;218(4):517-526. Epub 2012/08/14. https://doi.org/10.1016/j.imbio.2012.06.011. https://www.ncbi.nlm.nih.gov/pubmed/22884359
Shrot S, Katzav A, Korczyn AD, Litvinju Y, Hershenson R, Pick CG, Blank M, Zaech J, Shoenfeld Y, Sirota P, Chapman J. Behavioral and cognitive deficits occur only after prolonged exposure of mice to antiphospholipid antibodies. Lupus. 2002;11(11):736-743. Epub 2002/12/12. https://doi.org/10.1191/0961203302lu255oa. https://www.ncbi.nlm.nih.gov/pubmed/12475004
Bonfa E, Golombek SJ, Kaufman LD, Skelly S, Weissbach H, Brot N, Elkon KB. Association between lupus psychosis and anti-ribosomal P protein antibodies. The New England journal of medicine. 1987;317(5):265-271. Epub 1987/07/30. https://doi.org/10.1056/NEJM198707303170503. https://www.ncbi.nlm.nih.gov/pubmed/3496538
Abdel-Nasser AM, Ghaleb RM, Mahmoud JA, Khairy W, Mahmoud RM. Association of anti-ribosomal P protein antibodies with neuropsychiatric and other manifestations of systemic lupus erythematosus. Clinical rheumatology. 2008;27(11):1377-1385. Epub 2008/05/16. https://doi.org/10.1007/s10067-008-0921-1. https://www.ncbi.nlm.nih.gov/pubmed/18481154
Gonzalez A, Massardo L. Antibodies and the brain: antiribosomal P protein antibody and the clinical effects in patients with systemic lupus erythematosus. Current opinion in neurology. 2018. https://doi.org/10.1097/WCO.0000000000000549https://www.ncbi.nlm.nih.gov/pubmed/29461425.
Segovia-Miranda F, Serrano F, Dyrda A, Ampuero E, Retamal C, Bravo-Zehnder M, Parodi J, Zamorano P, Valenzuela D, Massardo L, van Zundert B, Inestrosa NC, Gonzalez A. Pathogenicity of lupus anti-ribosomal P antibodies: role of cross-reacting neuronal surface P antigen in glutamatergic transmission and plasticity in a mouse model. Arthritis Rheumatol. 2015;67(6):1598-1610. Epub 2015/02/25. https://doi.org/10.1002/art.39081. https://www.ncbi.nlm.nih.gov/pubmed/25709106
Elkon K, Skelly S, Parnassa A, Moller W, Danho W, Weissbach H, Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(19):7419-23. Epub 1986/10/01. https://doi.org/10.1073/pnas.83.19.7419. PubMed PMID: 2429305; PMCID: PMC386729. https://www.ncbi.nlm.nih.gov/pubmed/2429305
Elkon KB, Parnassa AP, Foster CL. Lupus autoantibodies target ribosomal P proteins. The Journal of experimental medicine. 1985;162(2):459-71. Epub 1985/08/01. https://doi.org/10.1084/jem.162.2.459. PubMed PMID: 2410526; PMCID: PMC2187754. https://www.ncbi.nlm.nih.gov/pubmed/2410526
•• Matus S, Burgos PV, Bravo-Zehnder M, Kraft R, Porras OH, Farias P, Barros LF, Torrealba F, Massardo L, Jacobelli S, Gonzalez A. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. The Journal of experimental medicine. 2007;204(13):3221-34. https://doi.org/10.1084/jem.20071285. PubMed PMID: 18056288; PMCID: 2150977. http://www.ncbi.nlm.nih.gov/pubmed/18056288. This study showed that anti-P antibodies recognize NSPA and cause rapid Ca2+ influx and apoptotic cell death in neurons.
Espinoza S, Arredondo SB, Barake F, Carvajal F, Guerrero FG, Segovia-Miranda F, Valenzuela DM, Wyneken U, Rojas-Fernandez A, Cerpa W, Massardo L, Varela-Nallar L, Gonzalez A. Neuronal surface P antigen (NSPA) modulates postsynaptic NMDAR stability through ubiquitination of tyrosine phosphatase PTPMEG. BMC Biol. 2020;18(1):164. Epub 2020/11/08. https://doi.org/10.1186/s12915-020-00877-2. PubMed PMID: 33158444; PMCID: PMC7648380. https://www.ncbi.nlm.nih.gov/pubmed/33158444
Bravo-Zehnder M, Toledo EM, Segovia-Miranda F, Serrano FG, Benito MJ, Metz C, et al. Anti-ribosomal P protein autoantibodies from patients with neuropsychiatric lupus impair memory in mice. Arthritis Rheumatol. 2015;67(1):204–14. https://doi.org/10.1002/art.38900https://www.ncbi.nlm.nih.gov/pubmed/25302407.
Katzav A, Solodeev I, Brodsky O, Chapman J, Pick CG, Blank M, Zhang W, Reichlin M, Shoenfeld Y. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis and rheumatism. 2007;56(3):938-948. Epub 2007/03/01. https://doi.org/10.1002/art.22419. https://www.ncbi.nlm.nih.gov/pubmed/17328071
Jeltsch-David H, Muller S. Neuropsychiatric systemic lupus erythematosus and cognitive dysfunction: the MRL-lpr mouse strain as a model. Autoimmunity reviews. 2014;13(9):963-973. Epub 2014/09/04. https://doi.org/10.1016/j.autrev.2014.08.015. https://www.ncbi.nlm.nih.gov/pubmed/25183233
Lauvsnes MB, Omdal R. Systemic lupus erythematosus, the brain, and anti-NR2 antibodies. Journal of neurology. 2012;259(4):622-629. Epub 2011/09/13. https://doi.org/10.1007/s00415-011-6232-5. http://www.ncbi.nlm.nih.gov/pubmed/21909801
Arinuma Y. Antibodies and the brain: anti-N-methyl-D-aspartate receptor antibody and the clinical effects in patients with systemic lupus erythematosus. Current opinion in neurology. 2018;31(3):294–9. https://doi.org/10.1097/WCO.0000000000000554https://www.ncbi.nlm.nih.gov/pubmed/29474315.
•• Arinuma Y, Yanagida T, Hirohata S. Association of cerebrospinal fluid anti-NR2 glutamate receptor antibodies with diffuse neuropsychiatric systemic lupus erythematosus. Arthritis and rheumatism. 2008;58(4):1130–5 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18383393. This study found that anti-dsDNA antibodies found in SLE CSF crossreact with NMDAR and induce apoptotic neuronal death.
DeGiorgio LA, Konstantinov KN, Lee SC, Hardin JA, Volpe BT, Diamond B. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nature medicine. 2001;7(11):1189–93.
Fujieda Y, Mader S, Jeganathan V, Arinuma Y, Shimizu Y, Kato M, Oku K, Minami A, Shimizu C, Yasuda S, Atsumi T. Clinical significance of anti-DNA/N-methyl-D-aspartate receptor 2 antibodies in de novo and post-steroid cases with neuropsychiatric systemic lupus erythematosus. Int J Rheum Dis. 2019;22(3):443-448. Epub 2018/10/26. https://doi.org/10.1111/1756-185X.13392. https://www.ncbi.nlm.nih.gov/pubmed/30358102
Gerosa M, Poletti B, Pregnolato F, Castellino G, Lafronza A, Silani V, Riboldi P, Meroni PL, Merrill JT. Antiglutamate receptor antibodies and cognitive impairment in primary antiphospholipid syndrome and systemic lupus erythematosus. Frontiers in immunology. 2016;7:5. Epub 2016/02/13. https://doi.org/10.3389/fimmu.2016.00005. PubMed PMID: 26870034; PMCID: PMC4740786. https://www.ncbi.nlm.nih.gov/pubmed/26870034
Gono T. Anti-NMDA receptor antibody in systemic lupus erythematosus. Nihon Shinkei Seishin Yakurigaku Zasshi. 2013;33(5-6):225–30 https://www.ncbi.nlm.nih.gov/pubmed/25069262.
Gono T, Kawaguchi Y, Kaneko H, Nishimura K, Hanaoka M, Kataoka S, Okamoto Y, Katsumata Y, Yamanaka H. Anti-NR2A antibody as a predictor for neuropsychiatric systemic lupus erythematosus. Rheumatology. 2011;50(9):1578-1585. Epub 2011/01/07. https://doi.org/10.1093/rheumatology/keq408. http://www.ncbi.nlm.nih.gov/pubmed/21208977
Gulati G, Iffland PH 2nd, Janigro D, Zhang B, Luggen ME. Anti-NR2 antibodies, blood-brain barrier, and cognitive dysfunction. Clinical rheumatology. 2016;35(12):2989–97. https://doi.org/10.1007/s10067-016-3339-1https://www.ncbi.nlm.nih.gov/pubmed/27357716.
•• Harrison MJ, Ravdin LD, Lockshin MD. Relationship between serum NR2a antibodies and cognitive dysfunction in systemic lupus erythematosus. Arthritis and rheumatism. 2006;54(8):2515–22. https://doi.org/10.1002/art.22030http://www.ncbi.nlm.nih.gov/pubmed/16868972. This study found anti-dsDNA/NMDAR antibodies in brains of SLE patients colocalized with NR2A, and cause neuronal death and cognitive dysfunction in mice.
• Kowal C, DeGiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proceedings of the National Academy of Sciences. 2006;103(52):19854–9. This study found serum anti-NR2 antibodies associated with depressive mood in SLE patients.
• Lapteva L, Nowak M, Yarboro CH, Takada K, Roebuck-Spencer T, Weickert T, et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis and rheumatism. 2006;54(8):2505–14. https://doi.org/10.1002/art.22031http://www.ncbi.nlm.nih.gov/pubmed/16868971. This study found an association of anti-NMDAR and anti-P antibodies with cognitive dysfunction in SLE patients with active disease.
Massardo L, Bravo-Zehnder M, Calderon J, Flores P, Padilla O, Aguirre JM, et al. Anti-N-methyl-D-aspartate receptor and anti-ribosomal-P autoantibodies contribute to cognitive dysfunction in systemic lupus erythematosus. Lupus. 2015;24(6):558–68. https://doi.org/10.1177/0961203314555538https://www.ncbi.nlm.nih.gov/pubmed/25318968.
Omdal R, Brokstad K, Waterloo K, Koldingsnes W, Jonsson R, Mellgren SI. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2005;12(5):392–8. https://doi.org/10.1111/j.1468-1331.2004.00976.xhttp://www.ncbi.nlm.nih.gov/pubmed/15804272.
Tay SH, Fairhurst AM, Mak A. Clinical utility of circulating anti-N-methyl-d-aspartate receptor subunits NR2A/B antibody for the diagnosis of neuropsychiatric syndromes in systemic lupus erythematosus and Sjogren's syndrome: An updated meta-analysis. Autoimmunity reviews. 2017;16(2):114–22. https://doi.org/10.1016/j.autrev.2016.12.002https://www.ncbi.nlm.nih.gov/pubmed/27988431.
Yoshio T, Onda K, Nara H, Minota S. Association of IgG anti-NR2 glutamate receptor antibodies in cerebrospinal fluid with neuropsychiatric systemic lupus erythematosus. Arthritis and rheumatism. 2006;54(2):675–8 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16447246.
•• Chang EH, Volpe BT, Mackay M, Aranow C, Watson P, Kowal C, et al. Selective impairment of spatial cognition caused by autoantibodies to the N-methyl-D-aspartate receptor. EBioMedicine. 2015;2(7):755–64. This study demonstrated that DNRAbs can also induce neuronal loss in the amygdala and emotional behavioral abnormalities when epinephrine is used to induce BBB permeability.
Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: antibodies alter emotion. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(3):678-83. Epub 2006/01/13. https://doi.org/10.1073/pnas.0510055103. PubMed PMID: 16407105; PMCID: 1334673. http://www.ncbi.nlm.nih.gov/pubmed/16407105
Katz JB, Limpanasithikul W, Diamond B. Mutational analysis of an autoantibody: differential binding and pathogenicity. The Journal of experimental medicine. 1994;180(3):925-32. PubMed PMID: 8064241; PMCID: 2191646. http://www.ncbi.nlm.nih.gov/pubmed/8064241
Kowal C, Degiorgio LA, Lee JY, Edgar MA, Huerta PT, Volpe BT, Diamond B. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(52):19854-9. Epub 2006/12/16. https://doi.org/10.1073/pnas.0608397104. PubMed PMID: 17170137; PMCID: 1702320. http://www.ncbi.nlm.nih.gov/pubmed/17170137
Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. The Journal of experimental medicine. 1998;188(1):29-38. PubMed PMID: 9653081; PMCID: 2525538. http://www.ncbi.nlm.nih.gov/pubmed/9653081
Schwarting A, Mockel T, Lutgendorf F, Triantafyllias K, Grella S, Boedecker S, Weinmann A, Meineck M, Sommer C, Schermuly I, Fellgiebel A, Luessi F, Weinmann-Menke J. Fatigue in SLE: diagnostic and pathogenic impact of anti-N-methyl-D-aspartate receptor (NMDAR) autoantibodies. Annals of the rheumatic diseases. 2019. Epub 2019/06/13. https://doi.org/10.1136/annrheumdis-2019-215098. https://www.ncbi.nlm.nih.gov/pubmed/31186256
Vo A, Volpe BT, Tang CC, Schiffer WK, Kowal C, Huerta PT, et al. Regional brain metabolism in a murine systemic lupus erythematosus model. Journal of Cerebral Blood Flow & Metabolism. 2014;34(8):1315–20.
Bodi N, Polgar A, Kiss E, Mester A, Poor G, Keri S. Reduced volumes of the CA1 and CA4-dentate gyrus hippocampal subfields in systemic lupus erythematosus. Lupus. 2017;26(13):1378–82. https://doi.org/10.1177/0961203317701845https://www.ncbi.nlm.nih.gov/pubmed/28355989.
Cannerfelt B, Nystedt J, Jonsen A, Latt J, van Westen D, Lilja A, Bengtsson A, Nilsson P, Martensson J, Sundgren PC. White matter lesions and brain atrophy in systemic lupus erythematosus patients: correlation to cognitive dysfunction in a cohort of systemic lupus erythematosus patients using different definition models for neuropsychiatric systemic lupus erythematosus. Lupus. 2018;27(7):1140-1149. Epub 2018/03/11. https://doi.org/10.1177/0961203318763533. https://www.ncbi.nlm.nih.gov/pubmed/29523054
Lapa AT, Pedro T, Francischinelli J, Coan AC, Costallat LT, Cendes F, Appenzeller S. Abnormality in hippocampal signal intensity predicts atrophy in patients with systemic lupus erythematosus. Lupus. 2017;26(6):633-639. Epub 2016/11/24. https://doi.org/10.1177/0961203316673151. https://www.ncbi.nlm.nih.gov/pubmed/27879427
Mackay M, Bussa MP, Aranow C, Ulug AM, Volpe BT, Huerta PT, Argyelan M, Mandel A, Hirsch J, Diamond B, Eidelberg D. Differences in regional brain activation patterns assessed by functional magnetic resonance imaging in patients with systemic lupus erythematosus stratified by disease duration. Molecular medicine. 2011;17(11-12):1349-56. https://doi.org/10.2119/molmed.2011.00185. PubMed PMID: 21953419; PMCID: 3321819. http://www.ncbi.nlm.nih.gov/pubmed/21953419
Mackay M, Tang CC, Volpe BT, Aranow C, Mattis PJ, Korff RA, Diamond B, Eidelberg D. Brain metabolism and autoantibody titres predict functional impairment in systemic lupus erythematosus. Lupus Sci Med. 2015;2(1):e000074. https://doi.org/10.1136/lupus-2014-000074. PubMed PMID: 25861456; PMCID: 4379887. http://www.ncbi.nlm.nih.gov/pubmed/25861456
Chang EH, Huerta PT. Neurophysiological correlates of object recognition in the dorsal subiculum. Frontiers in behavioral neuroscience. 2012;6:46. https://doi.org/10.3389/fnbeh.2012.00046. PubMed PMID: 22833721; PMCID: 3400129. http://www.ncbi.nlm.nih.gov/pubmed/22833721
•• Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, Volpe BT. Cognition and immunity; antibody impairs memory. Immunity. 2004;21(2):179-188. Epub 2004/08/17. https://doi.org/10.1016/j.immuni.2004.07.011. http://www.ncbi.nlm.nih.gov/pubmed/15308099. This study found that transient exposure to DNRAbs induces long term neuronal dysfunction mediated by activated microglia and C1q, which can be prevented with ACE inhibition in mice.
Nestor J, Arinuma Y, Huerta TS, Kowal C, Nasiri E, Kello N, Fujieda Y, Bialas A, Hammond T, Sriram U, Stevens B, Huerta PT, Volpe BT, Diamond B. Lupus antibodies induce behavioral changes mediated by microglia and blocked by ACE inhibitors. The Journal of experimental medicine. 2018;215(10):2554-66. Epub 2018/09/07. https://doi.org/10.1084/jem.20180776. PubMed PMID: 30185634; PMCID: PMC6170183. https://www.ncbi.nlm.nih.gov/pubmed/30185634
Kozora E, Brown MS, Filley CM, Zhang L, Miller DE, West SG, et al. Memory impairment associated with neurometabolic abnormalities of the hippocampus in patients with non-neuropsychiatric systemic lupus erythematosus. Lupus. 2011;20(6):598–606. https://doi.org/10.1177/0961203310392425https://www.ncbi.nlm.nih.gov/pubmed/21335397.
Ploran E, Tang C, Mackay M, Small M, Anderson E, Storbeck J, Bascetta B, Kang S, Aranow C, Sartori C, Watson P, Volpe B, Diamond B, Eidelberg D. Assessing cognitive impairment in SLE: examining relationships between resting glucose metabolism and anti-NMDAR antibodies with navigational performance. Lupus Sci Med. 2019;6(1):e000327. Epub 2019/08/16. https://doi.org/10.1136/lupus-2019-000327. PubMed PMID: 31413849; PMCID: PMC6667777. https://www.ncbi.nlm.nih.gov/pubmed/31413849
Roebuck-spencer TM, Yarboro C, Nowak M, Takada K, Jacobs G, Lapteva L, et al. Use of computerized assessment to predict neuropsychological functioning and emotional distress in patients with systemic lupus erythematosus. Arthritis Care & Research. 2006;55(3):434–41.
Zabala A, Salgueiro M, Saez-Atxukarro O, Ballesteros J, Ruiz-Irastorza G, Segarra R. Cognitive impairment in patients with neuropsychiatric and non-neuropsychiatric systemic lupus erythematosus: a systematic review and meta-analysis. Journal of the International Neuropsychological Society : JINS. 2018;24(6):629-639. Epub 2018/03/20. https://doi.org/10.1017/S1355617718000073. https://www.ncbi.nlm.nih.gov/pubmed/29553037
Zhu CM, Ma Y, Xie L, Huang JZ, Sun ZB, Duan SX, Lin ZR, Yin JJ, Le HB, Sun DM, Xu WC, Ma SH. Spatial working memory impairment in patients with non-neuropsychiatric systemic lupus erythematosus: a blood-oxygen-level dependent functional magnetic resonance imaging study. The Journal of rheumatology. 2017;44(2):201-208. Epub 2017/01/17. https://doi.org/10.3899/jrheum.160290. https://www.ncbi.nlm.nih.gov/pubmed/28089970
Brunner HI, Klein-Gitelman MS, Zelko F, Beebe DW, Foell D, Lee J, Zaal A, Jones J, Roebuck-Spencer T, Ying J. Blood-based candidate biomarkers of the presence of neuropsychiatric systemic lupus erythematosus in children. Lupus Sci Med. 2014;1(1):e000038. Epub 2014/11/15. https://doi.org/10.1136/lupus-2014-000038. PubMed PMID: 25396068; PMCID: PMC4225735. https://www.ncbi.nlm.nih.gov/pubmed/25396068
Husebye ES, Sthoeger ZM, Dayan M, Zinger H, Elbirt D, Levite M, et al. Autoantibodies to a NR2A peptide of the glutamate/NMDA receptor in sera of patients with systemic lupus erythematosus. Annals of the rheumatic diseases. 2005;64(8):1210–3 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15708887.
Rodriguez-Smith J, Brunner HI. Update on the treatment and outcome of systemic lupus erythematous in children. Curr Opin Rheumatol. 2019;31(5):464-470. Epub 2019/05/21. doi: https://doi.org/10.1097/BOR.0000000000000621. https://www.ncbi.nlm.nih.gov/pubmed/31107290
Lahita RG. Systemic lupus erythematosus: learning disability in the male offspring of female patients and relationship to laterality. Psychoneuroendocrinology. 1988;13(5):385–96 http://www.ncbi.nlm.nih.gov/pubmed/3205905.
Ross G, Sammaritano L, Nass R, Lockshin M. Effects of mothers' autoimmune disease during pregnancy on learning disabilities and hand preference in their children. Arch Pediatr Adolesc Med. 2003;157(4):397–402. https://doi.org/10.1001/archpedi.157.4.397https://www.ncbi.nlm.nih.gov/pubmed/12695238.
Tincani A, Nuzzo M, Motta M, Zatti S, Lojacono A, Faden D. Autoimmunity and pregnancy: autoantibodies and pregnancy in rheumatic diseases. Annals of the New York Academy of Sciences. 2006;1069:346-352. Epub 2006/07/21. doi: https://doi.org/10.1196/annals.1351.032. https://www.ncbi.nlm.nih.gov/pubmed/16855161
• Vinet E, Bernatsky S, Pineau CA, Clarke AE, Nashi EP, Scott S, et al. Increased male-to-female ratio among children born to women with systemic lupus erythematosus: comment on the article by Lockshin et al. Arthritis and rheumatism. 2013;65(4):1129. https://doi.org/10.1002/art.37852https://www.ncbi.nlm.nih.gov/pubmed/23335044. This group reviews the incidence and potential risk factors for stillbirth and neurodevelopmental disorders in children born to women with SLE, including in utero exposure to maternal autoantibodies and cytokine levels.
Vinet E, Genest G, Scott S, Pineau CA, Clarke AE, Platt RW, et al. Brief report: causes of stillbirths in women with systemic lupus erythematosus. Arthritis Rheumatol. 2016;68(10):2487–91. https://doi.org/10.1002/art.39742https://www.ncbi.nlm.nih.gov/pubmed/27159385.
Vinet E, Pineau CA, Clarke AE, Fombonne E, Platt RW, Bernatsky S. Neurodevelopmental disorders in children born to mothers with systemic lupus erythematosus. Lupus. 2014;23(11):1099–104. https://doi.org/10.1177/0961203314541691http://www.ncbi.nlm.nih.gov/pubmed/24969080.
Vinet E, Pineau CA, Clarke AE, Scott S, Fombonne E, Joseph L, et al. Increased risk of autism spectrum disorders in children born to women with systemic lupus erythematosus: results from a large population-based cohort. Arthritis Rheumatol. 2015;67(12):3201–8. https://doi.org/10.1002/art.39320https://www.ncbi.nlm.nih.gov/pubmed/26315754.
•• Yousef Yengej FA, van Royen-Kerkhof A, Derksen R, Fritsch-Stork RDE. The development of offspring from mothers with systemic lupus erythematosus. A systematic review. Autoimmunity reviews. 2017;16(7):701-711. Epub 2017/05/10. https://doi.org/10.1016/j.autrev.2017.05.005. https://www.ncbi.nlm.nih.gov/pubmed/28479488. This study in mice found significant behavioral alterations in males born to dams exposed to DNRAbs.
Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, Volpe BT. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15:91–6. https://doi.org/10.1038/nm.1892.
Wang L, Zhou D, Lee J, Niu H, Faust TW, Frattini S, Kowal C, Huerta PT, Volpe BT, Diamond B. Female mouse fetal loss mediated by maternal autoantibody. The Journal of experimental medicine. 2012;209(6):1083-9. https://doi.org/10.1084/jem.20111986. PubMed PMID: 22565825; PMCID: 3371726. http://www.ncbi.nlm.nih.gov/pubmed/22565825. This study in mice found increased loss of female embryos in pregnant mice exposed to DNRAbs.
Farmer K, Cady R, Reeves D, Bleiberg J. Cognitive efficiency following migraine therapy. Cephalalgia. 2001;21(4).
•• Wilken J, Kane R, Sullivan C, Wallin M, Usiskin J, Quig M, et al. The utility of computerized neuropsychological assessment of cognitive dysfunction in patients with relapsing-remitting multiple sclerosis. Multiple Sclerosis Journal. 2003;9(2):119–27. This study found that increased hippocampal hypermetabolism and DNRAb serum titers independently predicted poor memory performance, and together provided a more accurate prediction.
Mackay M, Tang CC, Volpe BT, Aranow C, Mattis PJ, Korff RA, et al. Brain metabolism and autoantibody titres predict functional impairment in systemic lupus erythematosus. Lupus Science & Medicine. 2015;2(1).
Costallat BL, Ferreira DM, Lapa AT, Rittner L, Costallat LTL, Appenzeller S. Brain diffusion tensor MRI in systematic lupus erythematosus: a systematic review. Autoimmunity reviews. 2018;17(1):36–43.
Brendel M, Focke C, Blume T, Peters F, Deussing M, Probst F, Jaworska A, Overhoff F, Albert N, Lindner S, von Ungern-Sternberg B, Bartenstein P, Haass C, Kleinberger G, Herms J, Rominger A. Time courses of cortical glucose metabolism and microglial activity across the life span of wild-type mice: a PET study. J Nucl Med. 2017;58(12):1984-1990. Epub 2017/07/15. https://doi.org/10.2967/jnumed.117.195107. https://www.ncbi.nlm.nih.gov/pubmed/28705919
Fan Z, Aman Y, Ahmed I, Chetelat G, Landeau B, Ray Chaudhuri K, Brooks DJ, Edison P. Influence of microglial activation on neuronal function in Alzheimer's and Parkinson's disease dementia. Alzheimers Dement. 2015;11(6):608-621 e7. Epub 2014/09/23. https://doi.org/10.1016/j.jalz.2014.06.016. https://www.ncbi.nlm.nih.gov/pubmed/25239737
Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36(10):587-97. Epub 2013/08/24. https://doi.org/10.1016/j.tins.2013.07.001. PubMed PMID: 23968694; PMCID: PMC3900881. https://www.ncbi.nlm.nih.gov/pubmed/23968694
Patel AB, Lai JC, Chowdhury GM, Hyder F, Rothman DL, Shulman RG, Behar KL. Direct evidence for activity-dependent glucose phosphorylation in neurons with implications for the astrocyte-to-neuron lactate shuttle. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(14):5385-90. Epub 2014/04/08. https://doi.org/10.1073/pnas.1403576111. PubMed PMID: 24706914; PMCID: PMC3986127. https://www.ncbi.nlm.nih.gov/pubmed/24706914
Ren T, Ho RCM, Mak A. Dysfunctional cortico–basal ganglia–thalamic circuit and altered hippocampal–amygdala activity on cognitive set-shifting in non-neuropsychiatric systemic lupus erythematosus. Arthritis & Rheumatism. 2012;64(12):4048–59.
Faust TW, Robbiati S, Huerta TS, Huerta PT. Dynamic NMDAR-mediated properties of place cells during the object place memory task. Frontiers in behavioral neuroscience. 2013;7:202.
Huerta PT, Gibson EL, Rey C, Huerta TS. Integrative neuroscience approach to neuropsychiatric lupus. Immunologic research. 2015;63(1-3):11–7.
Giannoccaro MP, Wright SK, Vincent A. In vivo mechanisms of antibody-mediated neurological disorders: animal models and potential implications. Front Neurol. 2019;10:1394. Epub 2020/03/03. https://doi.org/10.3389/fneur.2019.01394. PubMed PMID: 32116982; PMCID: PMC7013005. https://www.ncbi.nlm.nih.gov/pubmed/32116982
Chalmers SA, Wen J, Shum J, Doerner J, Herlitz L, Putterman C. CSF-1R inhibition attenuates renal and neuropsychiatric disease in murine lupus. Clinical immunology. 2017;185:100–8.
Telerman A, Lapter S, Sharabi A, Zinger H, Mozes E. Induction of hippocampal neurogenesis by a tolerogenic peptide that ameliorates lupus manifestations. Journal of neuroimmunology. 2011;232(1-2):151–7.
de Oliveira FF, Bertolucci PH, Chen ES, Smith MC. Brain-penetrating angiotensin-converting enzyme inhibitors and cognitive change in patients with dementia due to Alzheimer's disease. J Alzheimers Dis. 2014;42(Suppl 3):S321–4. https://doi.org/10.3233/JAD-132189https://www.ncbi.nlm.nih.gov/pubmed/24577465.
Hajjar IM, Keown M, Lewis P, Almor A. Angiotensin converting enzyme inhibitors and cognitive and functional decline in patients with Alzheimer's disease: an observational study. American Journal of Alzheimer's Disease & Other Dementias®. 2008;23(1):77–83.
O'Caoimh R, Healy L, Gao Y, Svendrovski A, Kerins DM, Eustace J, et al. Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with Alzheimer's disease. J Alzheimers Dis. 2014;40(3):595–603. https://doi.org/10.3233/JAD-131694https://www.ncbi.nlm.nih.gov/pubmed/24496072.
Soto ME, van Kan GA, Nourhashemi F, Gillette-Guyonnet S, Cesari M, Cantet C, et al. Angiotensin-converting enzyme inhibitors and Alzheimer's disease progression in older adults: results from the Reseau sur la Maladie d'Alzheimer Francais cohort. J Am Geriatr Soc. 2013;61(9):1482–8. https://doi.org/10.1111/jgs.12415https://www.ncbi.nlm.nih.gov/pubmed/24000874.
Yasar S, Xia J, Yao W, Furberg CD, Xue QL, Mercado CI, Fitzpatrick AL, Fried LP, Kawas CH, Sink KM, Williamson JD, DeKosky ST, Carlson MC, Ginkgo evaluation of memory study I. Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo Evaluation of Memory Study. Neurology. 2013;81(10):896-903. https://doi.org/10.1212/WNL.0b013e3182a35228. PubMed PMID: 23911756; PMCID: PMC3885216. https://www.ncbi.nlm.nih.gov/pubmed/23911756
Zhuang S, Wang HF, Wang X, Li J, Xing CM. The association of renin-angiotensin system blockade use with the risks of cognitive impairment of aging and Alzheimer's disease: A meta-analysis. J Clin Neurosci. 2016;33:32–8. https://doi.org/10.1016/j.jocn.2016.02.036https://www.ncbi.nlm.nih.gov/pubmed/27475317.
Torika N, Asraf K, Roasso E, Danon A, Fleisher-Berkovich S. Angiotensin converting enzyme inhibitors ameliorate brain inflammation associated with microglial activation: possible implications for Alzheimer’s disease. Journal of Neuroimmune Pharmacology. 2016;11(4):774–85.
Wright JW, Harding JW. The brain renin–angiotensin system: a diversity of functions and implications for CNS diseases. Pflügers Archiv-European Journal of Physiology. 2013;465(1):133–51.
Noda M, Kariura Y, Pannasch U, Nishikawa K, Wang L, Seike T, et al. Neuroprotective role of bradykinin because of the attenuation of pro-inflammatory cytokine release from activated microglia. Journal of neurochemistry. 2007;101(2):397–410.
Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. Journal of pharmacological sciences. 2005;99(1):6–38.
Asraf K, Torika N, Danon A, Fleisher-Berkovich S. Involvement of the bradykinin B1 receptor in microglial activation: in vitro and in vivo studies. Frontiers in Endocrinology. 2017;8:82.
Acknowledgement
The authors acknowledge support from grants from the National Institutes of Health (NIH P01AI073693).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Systemic Lupus Erythematosus
Rights and permissions
About this article
Cite this article
Zarfeshani, A., Carroll, K.R., Volpe, B.T. et al. Cognitive Impairment in SLE: Mechanisms and Therapeutic Approaches. Curr Rheumatol Rep 23, 25 (2021). https://doi.org/10.1007/s11926-021-00992-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s11926-021-00992-1