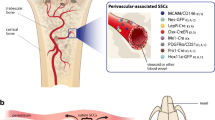
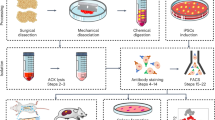
Abstract
Purpose of Review
The adult skeleton contains stem cells involved in growth, homeostasis, and healing. Mesenchymal or skeletal stem cells are proposed to provide precursors to osteoblasts, chondrocytes, marrow adipocytes, and stromal cells. We review the evidence for existence and functionality of different skeletal stem cell pools, and the tools available for identifying or targeting these populations in mouse and human tissues.
Recent Findings
Lineage tracing and single cell-based techniques in mouse models indicate that multiple pools of stem cells exist in postnatal bone. These include growth plate stem cells, stem and progenitor cells in the diaphysis, reticular cells that only form bone in response to injury, and injury-responsive periosteal stem cells. New staining protocols have also been described for prospective isolation of human skeletal stem cells.
Summary
Several populations of postnatal skeletal stem and progenitor cells have been identified in mice, and we have an increasing array of tools to target these cells. Most Cre models lack a high degree of specificity to define single populations. Human studies are less advanced and require further efforts to refine methods for identifying stem and progenitor cells in adult bone.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. https://doi.org/10.1038/nm.3028.
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–13. https://doi.org/10.1016/j.stem.2008.07.003.
Sipp D, Robey PG, Turner L. Clear up this stem-cell mess. Nature. 2018;561(7724):455–7. https://doi.org/10.1038/d41586-018-06756-9.
Bragdon BC, Bahney CS. Origin of reparative stem cells in fracture healing. Curr Osteoporos Rep. 2018;16(4):490–503. https://doi.org/10.1007/s11914-018-0458-4.
• de Lageneste Duchamp O, Julien A, Abou-Khalil R, Frangi G, Carvalho C, Cagnard N, et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun. 2018;9(1):773. https://doi.org/10.1038/s41467-018-03124-zShowed the high potential of periosteal SSC in skeletal regeneration, which requires the presence of periostin.
Ono N, Ono W, Nagasawa T, Kronenberg HM. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol. 2014;16(12):1157–67. https://doi.org/10.1038/ncb3067.
Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15(2):154–68. https://doi.org/10.1016/j.stem.2014.06.008.
Matsushita Y, Ono W, Ono N. Skeletal stem cells for bone development and repair: diversity matters. Curr Osteoporos Rep. 2020;18(3):189–98. https://doi.org/10.1007/s11914-020-00572-9.
• Pineault KM, Song JY, Kozloff KM, Lucas D, Wellik DM. Hox11 expressing regional skeletal stem cells are progenitors for osteoblasts, chondrocytes and adipocytes throughout life. Nat Commun. 2019;10(1):3168. https://doi.org/10.1038/s41467-019-11100-4Develop Hoxa11-CreER and show that cells labeled in development or early postnatal life continue to contribute to osteoblast formation for many months specifically in the zeugopod limbs, confirming that progenitors involved in growth, turnover, and healing do not travel from other bones.
Matthews BG, Ono N, Kalaizic I. Methods in lineage tracing. Principles of Bone Biology. Academic Press; 2020. p. 1887–98.
He X, Bougioukli S, Ortega B, Arevalo E, Lieberman JR, McMahon AP. Sox9 positive periosteal cells in fracture repair of the adult mammalian long bone. Bone. 2017;103:12–9. https://doi.org/10.1016/j.bone.2017.06.008.
Kuwahara ST, Serowoky MA, Vakhshori V, Tripuraneni N, Hegde NV, Lieberman JR, et al. Sox9+ messenger cells orchestrate large-scale skeletal regeneration in the mammalian rib. eLife. 2019;8:e40715. https://doi.org/10.7554/eLife.40715.
Balani DH, Ono N, Kronenberg HM. Parathyroid hormone regulates fates of murine osteoblast precursors in vivo. J Clin Invest. 2017;127(9):3327–38. https://doi.org/10.1172/JCI91699.
•• Mizuhashi K, Ono W, Matsushita Y, Sakagami N, Takahashi A, Saunders TL, et al. Resting zone of the growth plate houses a unique class of skeletal stem cells. Nature. 2018;563(7730):254–8. https://doi.org/10.1038/s41586-018-0662-5Established Pthrp-CreER as a marker of a subset of resting growth plate cells and demonstrate their capacity to form chondrocytes that go on to become osteoblasts and stromal cells but not adipocytes in the trabecular area.
Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160(1–2):269–84.
Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, et al. Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell. 2016;19(5):628–42. https://doi.org/10.1016/j.stem.2016.08.001.
Brownell I, Guevara E, Bai CB, Loomis CA, Joyner AL. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell. 2011;8(5):552–65. https://doi.org/10.1016/j.stem.2011.02.021.
•• Shi Y, He G, Lee WC, McKenzie JA, Silva MJ, Long F. Gli1 identifies osteogenic progenitors for bone formation and fracture repair. Nat Commun. 2017;8(1):2043. https://doi.org/10.1038/s41467-017-02171-2Characterization of Gli1-CreER labeled cells in both development and postnatal bone, and demonstration of their functional role in trabecular bone accrual.
Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29:340–9.
Liu YL, Strecker S, Wang LP, Kronenberg MS, Wang W, Rowe DW, et al. Osterix-Cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PLoS One. 2013;8(8):e71318. https://doi.org/10.1371/journal.pone.0071318.
Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10(3):259–72. https://doi.org/10.1016/j.stem.2012.02.003.
• Ortinau LC, Wang H, Lei K, Deveza L, Jeong Y, Hara Y, et al. Identification of functionally distinct Mx1+alphaSMA+ periosteal skeletal stem cells. Cell Stem Cell. 2019;25(6):784–96 e5. https://doi.org/10.1016/j.stem.2019.11.003Show that Mx1-Cre targets periosteal stem cells, and that αSMA-GFP enriches for stem-like populations, at least ex vivo. Identify CCL5 as a mediator of migration.
Song JY, Pineault KM, Dones JM, Raines RT, Wellik DM. Hox genes maintain critical roles in the adult skeleton. Proc Natl Acad Sci U S A. 2020;117(13):7296–304. https://doi.org/10.1073/pnas.1920860117.
•• Matsushita Y, Nagata M, Kozloff KM, Welch JD, Mizuhashi K, Tokavanich N, et al. A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration. Nat Commun. 2020;11(1):332. https://doi.org/10.1038/s41467-019-14029-wCharacterise functionality of Cxcl12+ central marrow stromal cells using Cxcl12-CreER. Under homeostatic conditions they demonstrate, they do not spread far within the marrow, or form osteoblasts, but become more mobile and osteogenic following injury. Clearly demonstrate heterogeneity of cells involved in injury reponse including those that do not normally contribute to the osteoblast pool.
• Seike M, Omatsu Y, Watanabe H, Kondoh G, Nagasawa T. Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes Dev. 2018;32(5–6):359–72. https://doi.org/10.1101/gad.311068.117Establish Ebf3-CreER and examine Ebf3 function in the bone marrow. Lineage tracing data is minimal, and we look forward to more detailed characterization of this model, but functional characterization is striking indicating that Ebf3 is important for inhibiting bone formation in the marrow cavity.
Matic I, Matthews BG, Wang X, Dyment NA, Worthley DL, Rowe DW, et al. Quiescent bone lining cells are a major source of osteoblasts during adulthood. Stem Cells. 2016;34(12):2930–42. https://doi.org/10.1002/stem.2474.
Grcevic D, Pejda S, Matthews BG, Repic D, Wang LP, Li HT, et al. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells. 2012;30(2):187–96. https://doi.org/10.1002/stem.780.
Matthews BG, Sbrana FV, Novak S, Funnell JL, Cao Y, Buckels EJ, et al. Heterogeneity of murine periosteum osteochondroprogenitors involved in fracture healing. bioRxiv. 2020:2020.06.24.169003. https://doi.org/10.1101/2020.06.24.169003.
Prater MD, Petit V, Alasdair Russell I, Giraddi RR, Shehata M, Menon S, et al. Mammary stem cells have myoepithelial cell properties. Nat Cell Biol. 2014;16(10):942–50. https://doi.org/10.1038/ncb3025.
Kawanami A, Matsushita T, Chan YY, Murakami S. Mice expressing GFP and CreER in osteochondro progenitor cells in the periosteum. Biochem Biophys Res Commun. 2009;386(3):477–82. https://doi.org/10.1016/j.bbrc.2009.06.059.
Moore ER, Yang Y, Jacobs CR. Primary cilia are necessary for Prx1-expressing cells to contribute to postnatal skeletogenesis. J Cell Sci. 2018;131(16):jcs217828. https://doi.org/10.1242/jcs.217828.
Rux DR, Song JY, Swinehart IT, Pineault KM, Schlientz AJ, Trulik KG, et al. Regionally restricted Hox function in adult bone marrow multipotent mesenchymal stem/stromal cells. Dev Cell. 2016;39(6):653–66. https://doi.org/10.1016/j.devcel.2016.11.008.
Matsushita Y, Ono W, Ono N. Growth plate skeletal stem cells and their transition from cartilage to bone. Bone. 2020;136:115359. https://doi.org/10.1016/j.bone.2020.115359.
Wolff LI, Hartmann C. A second career for chondrocytes—transformation into osteoblasts. Current Osteoporosis Reports. 2019;17(3):129–37. https://doi.org/10.1007/s11914-019-00511-3.
Chan Charles KF, Seo Eun Y, Chen James Y, Lo D, McArdle A, Sinha R, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160(1–2):285–98. https://doi.org/10.1016/j.cell.2014.12.002.
Zhao H, Feng J, Seidel K, Shi S, Klein O, Sharpe P, et al. Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell. 2014;14(2):160–73. https://doi.org/10.1016/j.stem.2013.12.013.
Zhao H, Feng JF, Ho TV, Grimes W, Urata M, Chai Y. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat Cell Biol. 2015;17(4):386. https://doi.org/10.1038/ncb3139.
Wang W, Strecker S, Liu Y, Wang L, Assanah F, Smith S, et al. Connective tissue growth factor reporter mice label a subpopulation of mesenchymal progenitor cells that reside in the trabecular bone region. Bone. 2015;71:76–88. https://doi.org/10.1016/j.bone.2014.10.005.
Kim SW, Lu Y, Williams EA, Lai F, Lee JY, Enishi T, et al. Sclerostin antibody administration converts bone lining cells into active osteoblasts. J Bone Miner Res. 2017;32(5):892–901. https://doi.org/10.1002/jbmr.3038.
Kim SW, Pajevic PD, Selig M, Barry KJ, Yang JY, Shin CS, et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res. 2012;27(10):2075–84. https://doi.org/10.1002/jbmr.1665.
Root SH, Wee NKY, Novak S, Rosen CJ, Baron R, Matthews BG, et al. Perivascular osteoprogenitors are associated with transcortical channels of long bones. Stem Cells. 2020;38:769–81. https://doi.org/10.1002/stem.3159.
•• Zhong L, Yao L, Tower RJ, Wei Y, Miao Z, Park J, et al. Single cell transcriptomics identifies a unique adipose lineage cell population that regulates bone marrow environment. Elife. 2020;9:e54695. https://doi.org/10.7554/eLife.54695Present single cell RNAseq data from bone marrow and endosteal cells, clearing mapping out different stromal populations and showing expression of most of the previously proposed markers in these populations. Discover cells with adipocyte gene signature that are preadipogenic reticular cells that inhibit bone formation that appear to greatly overlap with populations including LepR-Cre+ cells.
Joseph C, Quach JM, Walkley CR, Lane SW, Lo Celso C, Purton LE. Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell Stem Cell. 2013;13:520–33.
Wang L, Tower RJ, Chandra A, Yao L, Tong W, Xiong Z, et al. Periosteal mesenchymal progenitor dysfunction and extraskeletally-derived fibrosis contribute to atrophic fracture nonunion. J Bone Miner Res. 2019;34(3):520–32. https://doi.org/10.1002/jbmr.3626.
•• Debnath S, Yallowitz AR, McCormick J, Lalani S, Zhang T, Xu R, et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562(7725):133–9. https://doi.org/10.1038/s41586-018-0554-8Identify periosteal stem cells using a combination of lineage markers and cell surface markers.
Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24(2):274–82. https://doi.org/10.1359/jbmr.081003.
Wang T, Zhang X, Bikle DD. Osteogenic differentiation of periosteal cells during fracture healing. J Cell Physiol. 2016;232:913–21. https://doi.org/10.1002/jcp.25641.
Kolind M, Bobyn JD, Matthews BG, Mikulec K, Aiken A, Little DG, et al. Lineage tracking of mesenchymal and endothelial progenitors in BMP-induced bone formation. Bone. 2015;81:53–9. https://doi.org/10.1016/j.bone.2015.06.023.
Matthews BG, Grcevic D, Wang L, Hagiwara Y, Roguljic H, Joshi P, et al. Analysis of alphaSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing. J Bone Miner Res. 2014;29(5):1283–94. https://doi.org/10.1002/jbmr.2140.
Hu X, Garcia M, Weng L, Jung X, Murakami JL, Kumar B, et al. Identification of a common mesenchymal stromal progenitor for the adult haematopoietic niche. Nat Commun. 2016;7:13095. https://doi.org/10.1038/ncomms13095.
Boulais PE, Mizoguchi T, Zimmerman S, Nakahara F, Vivie J, Mar JC, et al. The majority of CD45– TER119– CD31– bone marrow cell fraction is of hematopoietic origin and contains erythroid and lymphoid progenitors. Immunity. 2018;49:627–39.
Nakamura Y, Arai F, Iwasaki H, Hosokawa K, Kobayashi I, Gomei Y, et al. Isolation and characterization of endosteal niche cell populations that regulate hematopoietic stem cells. Blood. 2010;116(9):1422–32. https://doi.org/10.1182/blood-2009-08-239194.
Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–96. https://doi.org/10.1084/jem.20091046.
•• Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20(6):771–84 e6. https://doi.org/10.1016/j.stem.2017.02.009Thorough characterization of bone marrow populations isolated based on expression of PDGFRα, Sca1, and CD24. Find populations that are multipotent, osteo-chondral, and adipocyte progenitors as well as more committed preadipocytes.
Lundberg P, Allison SJ, Lee NJ, Baldock PA, Brouard N, Rost S, et al. Greater bone formation of Y2 knockout mice is associated with increased osteoprogenitor numbers and altered Y1 receptor expression. J Biol Chem. 2007;282(26):19082–91. https://doi.org/10.1074/jbc.M609629200.
Arthur A, Nguyen TM, Paton S, Zannettino ACW, Gronthos S. Loss of EfnB1 in the osteogenic lineage compromises their capacity to support hematopoietic stem/progenitor cell maintenance. Exp Hematol. 2019;69:43–53. https://doi.org/10.1016/j.exphem.2018.10.004.
Winkler IG, Barbier V, Wadley R, Zannettino ACW, Williams S, Levesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116(3):375–85. https://doi.org/10.1182/Blood-2009-07-233437.
Anjos-Afonso F, Bonnet D. Prospective identification and isolation of murine bone marrow derived multipotent mesenchymal progenitor cells. Best Pract Res Clin Haematol. 2011;24(1):13–24. https://doi.org/10.1016/j.beha.2010.11.003.
Pinho S, Lacombe J, Hanoun M, Mizoguchi T, Bruns I, Kunisaki Y, et al. PDGFR alpha and CD51 mark human Nestin(+) sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. J Exp Med. 2013;210(7):1351–67. https://doi.org/10.1084/jem.20122252.
Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. https://doi.org/10.1038/nature11885.
Abbuehl JP, Tatarova Z, Held W, Huelsken J. Long-term engraftment of primary bone marrow stromal cells repairs niche damage and improves hematopoietic stem cell transplantation. Cell Stem Cell. 2017;21(2):241–55.e6. https://doi.org/10.1016/j.stem.2017.07.004.
Li H, Ghazanfari R, Zacharaki D, Ditzel N, Isern J, Ekblom M, et al. Low/negative expression of PDGFR-α identifies the candidate primary mesenchymal stromal cells in adult human bone marrow. Stem Cell Reports. 2014;3:965–74.
Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457(7228):490–4. https://doi.org/10.1038/nature07547.
Chan CKF, Lindau P, Jiang W, Chen JY, Zhang LF, Chen C-C, et al. Clonal precursor of bone, cartilage, and hematopoietic niche stromal cells. Proc Natl Acad Sci. 2013;110(31):12643–8. https://doi.org/10.1073/pnas.1310212110.
Marecic O, Tevlin R, McArdle A, Seo EY, Wearda T, Duldulao C, et al. Identification and characterization of an injury-induced skeletal progenitor. Proc Natl Acad Sci U S A. 2015;112(32):9920–5. https://doi.org/10.1073/pnas.1513066112.
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. https://doi.org/10.1038/nature09262.
Tournaire G, Stegen S, Giacomini G, Stockmans I, Moermans K, Carmeliet G, et al. Nestin-GFP transgene labels skeletal progenitors in the periosteum. Bone. 2020;133:115259.
Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62. https://doi.org/10.1038/nature10783.
Ono N, Ono W, Mizoguchi T, Nagasawa T, Frenette PS, Kronenberg HM. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev Cell. 2014;29:330–9.
Mabuchi Y, Matsuzaki Y. Prospective isolation of resident adult human mesenchymal stem cell population from multiple organs. Int J Hematol. 2016;103(2):138–44. https://doi.org/10.1007/s12185-015-1921-y.
Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32(6):1408–19. https://doi.org/10.1002/stem.1681.
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36. https://doi.org/10.1016/j.cell.2007.08.025.
Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22(11):2903–11. https://doi.org/10.1093/humrep/dem265.
Paul G, Ozen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E, et al. The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS One. 2012;7(4):e35577. https://doi.org/10.1371/journal.pone.0035577.
Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. https://doi.org/10.1359/jbmr.2003.18.4.696.
Applebaum M, Kalcheim C. Mechanisms of myogenic specification and patterning. Results Probl Cell Differ. 2015;56:77–98. https://doi.org/10.1007/978-3-662-44608-9_4.
Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, et al. No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Reports. 2016;6(6):897–913. https://doi.org/10.1016/j.stemcr.2016.05.011.
Tormin A, Li O, Brune JC, Walsh S, Schutz B, Ehinger M, et al. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117(19):5067–77. https://doi.org/10.1182/blood-2010-08-304287.
•• Chan CKF, Gulati GS, Sinha R, Tompkins JV, Lopez M, Carter AC, et al. Identification of the human skeletal stem cell. Cell. 2018;175(1):43–56 e21. https://doi.org/10.1016/j.cell.2018.07.029They established novel staining protocols to isolate skeletal stem cells from human tissue. They focused on the pre-hypertrophic zone of the growth plate in fetal tissue as the main source of these cells, which does not completely align with the mouse data, but the study is impressive nonetheless.
Funding
This work has been supported by the Health Research Council of New Zealand Sir Charles Hercus Fellowship, the Auckland Medical Research Foundation, and the American Society for Bone and Mineral Research Rising Star Award to BGM. YC is supported by a University of Auckland Doctoral Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Skeletal Biology and Regulation
Rights and permissions
About this article
Cite this article
Cao, Y., Buckels, E.J. & Matthews, B.G. Markers for Identification of Postnatal Skeletal Stem Cells In Vivo. Curr Osteoporos Rep 18, 655–665 (2020). https://doi.org/10.1007/s11914-020-00622-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-020-00622-2