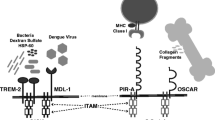
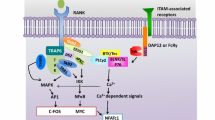
Abstract
It is important to understand the molecular mechanisms regulating osteoclast formation, as excess activation of osteoclasts is associated with various osteopenic disorders. Receptor activator of nuclear factor kappa B (RANKL) is a central player in osteoclastogenesis. Recent findings suggest that osteocytes are the major supplier of RANKL to osteoclast precursors. It has also been suggested that osteocyte cell death upregulates the RANKL/osteoprotegerin (OPG) ratio in viable osteocytes adjacent to apoptotic osteocytes in areas of bone microdamage, thus, contributing to localized osteoclast formation. Indeed, viable osteocytes can provide RANKL through direct interactions with osteoclast precursors at osteocyte dendritic processes. In addition, OPG tightly regulates RANKL cell surface presentation in osteocytes, which contributes to the inhibition of RANKL signaling, as well as the decoy receptor function of OPG. By contrast, the physiological role of RANKL in osteoblasts is yet to be clarified, although similar mechanisms of regulation are observed in both osteocytes and osteoblasts.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–96. doi:10.1196/annals.1365.035.
Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8(3):147–59.
Martin TJ, Ng KW. Mechanisms by which cells of the osteoblast lineage control osteoclast formation and activity. J Cell Biochem. 1994;56(3):357–66. doi:10.1002/jcb.240560312.
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–24.
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–23.
Trambas CM, Griffiths GM. Delivering the kiss of death. Nat Immunol. 2003;4(5):399–403.
Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, et al. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19(7):1059–66. doi:10.1359/JBMR.040305.
Silva I, Branco JC. Denosumab: recent update in postmenopausal osteoporosis. Acta Reumatologica Portuguesa. 2012;37(4):302–13.
Lewiecki EM, Miller PD, McClung MR, Cohen SB, Bolognese MA, Liu Y, et al. Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res. 2007;22(12):1832–41.
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901.
Tanaka S, Nakamura I, Inoue J, Oda H, Nakamura K. Signal transduction pathways regulating osteoclast differentiation and function. J Bone Miner Metab. 2003;21(3):123–33. doi:10.1007/s007740300021.
Hikita A, Kadono Y, Chikuda H, Fukuda A, Wakeyama H, Yasuda H, et al. Identification of an alternatively spliced variant of Ca2+-promoted Ras inactivator as a possible regulator of RANKL shedding. J Biol Chem. 2005;280(50):41700–6.
Wong BR, Josien R, Lee SY, Vologodskaia M, Steinman RM, Choi Y. The TRAF family of signal transducers mediates NF-kappaB activation by the TRANCE receptor. J Biol Chem. 1998;273(43):28355–9.
Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, et al. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells. 1999;4(6):353–62.
Tanaka S, Nakamura K, Takahasi N, Suda T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev. 2005;208:30–49. doi:10.1111/j.0105-2896.2005.00327.x.
Huang H, Chang EJ, Ryu J, Lee ZH, Lee Y, Kim HH. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem Biophys Res Commun. 2006;351(1):99–105.
Kim K, Kim JH, Lee J, Jin HM, Lee SH, Fisher DE, et al. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J Biol Chem. 2005;280(42):35209–16. doi:10.1074/jbc.M505815200.
Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, et al. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem. 2004;279(25):26475–80. doi:10.1074/jbc.M313973200.
Crotti TN, Flannery M, Walsh NC, Fleming JD, Goldring SR, McHugh KP. NFATc1 regulation of the human beta3 integrin promoter in osteoclast differentiation. Gene. 2006;372:92–102. doi:10.1016/j.gene.2005.12.012.
Jimi E, Nakamura I, Amano H, Taguchi Y, Tsurukai T, Tamura M, et al. Osteoclast function is activated by osteoblastic cells through a mechanism involving cell-to-cell contact. Endocrinology. 1996;137(8):2187–90.
Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an osteocyte-like cell line, MLO-Y4. J Bone Miner Res. 1997;12(12):2014–23. doi:10.1359/jbmr.1997.12.12.2014.
Zhao S, Zhang YK, Harris S, Ahuja SS, Bonewald LF. MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res. 2002;17(11):2068–79. doi:10.1359/jbmr.2002.17.11.2068.
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–4. doi:10.1038/nm.2452. This study showed that osteocytes are the major source of RANKL in physiological osteoclastogenesis.
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235–41. doi:10.1038/nm.2448. This study showed that osteocytes are the major source of RANKL in physiological osteoclastogenesis.
Cardoso L, Herman BC, Verborgt O, Laudier D, Majeska RJ, Schaffler MB. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009;24(4):597–605. doi:10.1359/jbmr.081210.
Herman BC, Cardoso L, Majeska RJ, Jepsen KJ, Schaffler MB. Activation of bone remodeling after fatigue: differential response to linear microcracks and diffuse damage. Bone. 2010;47(4):766–72. doi:10.1016/j.bone.2010.07.006.
Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metabol. 2007;5(6):464–75. doi:10.1016/j.cmet.2007.05.001.
Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012;50(5):1115–22. doi:10.1016/j.bone.2012.01.025. This study showed that RANKL/OPG ratio is up-regulated in osteocytes adjacent to the damaged site of bone.
Tomkinson A, Reeve J, Shaw RW, Noble BS. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82(9):3128–35.
Franz-Odendaal TA, Hall BK, Witten PE. Buried alive: how osteoblasts become osteocytes. Dev Dyn. 2006;235(1):176–90. doi:10.1002/dvdy.20603.
Kamioka H, Honjo T, Takano-Yamamoto T. A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone. 2001;28(2):145–9.
Honma M, Ikebuchi Y, Kariya Y, Hayashi M, Hayashi N, Aoki S, et al. RANKL subcellular trafficking and regulatory mechanisms in osteocytes. J Bone Miner Res. 2013;28(9):1936–49. doi:10.1002/jbmr.1941. This study showed that osteocytes provide RANKL to osteoclast precursors through direct cell-cell interactions.
Honma M, Ikebuchi Y, Kariya Y, Suzuki H. Establishment of optimized in vitro assay methods for evaluating osteocyte functions. J Bone Miner Metab. 2014. doi:10.1007/s00774-013-0555-5.
Kariya Y, Honma M, Aoki S, Chiba A, Suzuki H. Vps33a mediates RANKL storage in secretory lysosomes in osteoblastic cells. J Bone Miner Res. 2009;24(10):1741–52. doi:10.1359/jbmr.090409.
Aoki S, Honma M, Kariya Y, Nakamichi Y, Ninomiya T, Takahashi N, et al. Function of OPG as a traffic regulator for RANKL is crucial for controlled osteoclastogenesis. J Bone Miner Res. 2010;25(9):1907–21. doi:10.1002/jbmr.89.
Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65(18):2801–13.
Blott EJ, Griffiths GM. Secretory lysosomes. Nat Rev Mol Cell Biol. 2002;3(2):122–31.
Kariya Y, Honma M, Hanamura A, Aoki S, Ninomiya T, Nakamichi Y, et al. Rab27a and Rab27b are involved in stimulation-dependent RANKL release from secretory lysosomes in osteoblastic cells. J Bone Miner Res. 2010. doi:10.1002/jbmr.268.
Chavas L, Ihara K, Kawasaki M, Torii S, Uejima T, Kato R, et al. Elucidation of Rab27 recruitment by its effectors: structure of Rab27a bound to Exophilin4/Slp2-a. Structure. 2008;16(10):1468–77.
Hume AN, Ushakov DS, Tarafder AK, Ferenczi MA, Seabra MC. Rab27a and MyoVa are the primary Mlph interactors regulating melanosome transport in melanocytes. J Cell Sci. 2007;120(Pt 17):3111–22.
Stinchcombe J, Barral D, Mules E, Booth S, Hume A, Machesky L, et al. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152(4):825–34.
Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15(5):751–61.
Barral D, Ramalho J, Anders R, Hume A, Knapton H, Tolmachova T, et al. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J Clin Invest. 2002;110(2):247–57.
Fukuda M. Rab27 and its effectors in secretory granule exocytosis: a novel docking machinery composed of a Rab27.effector complex. Biochem Soc Trans. 2006;34(Pt 5):691–5.
Compliance with Ethics Guidelines
Conflict of Interest
M. Honma has received research support from Grant-in-Aid for Scientific Research (B) 24390349 from the Japan Society for the Promotion of Science. Y. Ikebuchi has received research support from Grant-in-Aid for Challenging Exploratory Research 25670632 from the Japan Society for the Promotion of Science. Y. Kariya has received research support from Grant-in-Aid for Young Scientists (Start-up) 24890048 from the Japan Society for the Promotion of Science. H Suzuki declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
All studies by M. Honma, Y. Ikebuchi, Y. Kariya and H. Suzuki involving animal subjects were performed after approval of the Institutional Animal Care and Use Committee of Graduate School of Medicine, the University of Tokyo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Honma, M., Ikebuchi, Y., Kariya, Y. et al. Regulatory Mechanisms of RANKL Presentation to Osteoclast Precursors. Curr Osteoporos Rep 12, 115–120 (2014). https://doi.org/10.1007/s11914-014-0189-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-014-0189-0