Abstract
Purpose of Review
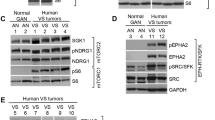
Neurofibromatosis 2 (NF2) is an autosomal-dominant genetic disorder characterized by bilateral vestibular schwannomas (VS), meningiomas, ependymomas, spinal and peripheral schwannomas, optic gliomas, and juvenile cataracts. Ongoing studies provide new insight into the role of the NF2 gene and merlin in VS tumorigenesis.
Recent Findings
As NF2 tumor biology becomes increasingly understood, therapeutics targeting specific molecular pathways have been developed and evaluated in preclinical and clinical studies.
Summary
NF2-associated VS are a source of significant morbidity with current treatments including surgery, radiation, and observation. Currently, there are no FDA-approved medical therapies for VS, and the development of selective therapeutics is a high priority. This manuscript reviews NF2 tumor biology and current therapeutics undergoing investigation for treatment of patients with VS.
Similar content being viewed by others
References
Asthagiri AR, Parry DM, Butman JA, Kim HJ, Tsilou ET, Zhuang Z, et al. Neurofibromatosis type 2. Lancet. 2009;373(9679):1974–86. S0140–6736(09)60259–2 [pii] https://doi.org/10.1016/S0140-6736(09)60259-2.
Di Maio S, Akagami R. Prospective comparison of quality of life before and after observation, radiation, or surgery for vestibular schwannomas. J Neurosurg. 2009;111(4):855–62. https://doi.org/10.3171/2008.10.Jns081014.
Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72(5):791–800. https://doi.org/10.1016/0092-8674(93)90406-g.
McClatchey AI, Giovannini M. Membrane organization and tumorigenesis–the NF2 tumor suppressor. Merlin Genes Dev. 2005;19(19):2265–77. https://doi.org/10.1101/gad.1335605.
Hung G, Li X, Faudoa R, Xeu Z, Kluwe L, Rhim JS, et al. Establishment and characterization of a schwannoma cell line from a patient with neurofibromatosis 2. Int J Oncol. 2002;20(3):475–82.
Giovannini M, Robanus-Maandag E, van der Valk M, Niwa-Kawakita M, Abramowski V, Goutebroze L, et al. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 2000;14(13):1617–30.
Gehlhausen JR, Park SJ, Hickox AE, Shew M, Staser K, Rhodes SD, et al. A murine model of neurofibromatosis type 2 that accurately phenocopies human schwannoma formation. Hum Mol Genet. 2015;24(1):1–8. https://doi.org/10.1093/hmg/ddu414.
Plotkin SR, Stemmer-Rachamimov AO, Barker FG, 2nd, Halpin C, Padera TP, Tyrrell A, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med. 2009;361(4):358–67. NEJMoa0902579 [pii] https://doi.org/10.1056/NEJMoa0902579.
Blakeley JO, Ye X, Duda DG, Halpin CF, Bergner AL, Muzikansky A, et al. Efficacy and biomarker study of bevacizumab for hearing loss resulting from neurofibromatosis type 2-associated vestibular schwannomas. J Clin Oncol. 2016;34(14):1669–75. https://doi.org/10.1200/JCO.2015.64.3817.
Synodos for NFC, Allaway R, Angus SP, Beauchamp RL, Blakeley JO, Bott M, et al. Traditional and systems biology based drug discovery for the rare tumor syndrome neurofibromatosis type 2. PLoS One. 2018;13(6):e0197350. https://doi.org/10.1371/journal.pone.0197350.
Chang LS, Oblinger JL, Smith AE, Ferrer M, Angus SP, Hawley E, et al. Brigatinib causes tumor shrinkage in both NF2-deficient meningioma and schwannoma through inhibition of multiple tyrosine kinases but not ALK. PLoS One. 2021;16(7):e0252048. https://doi.org/10.1371/journal.pone.0252048.
Plotkin SR, Halpin C, McKenna MJ, Loeffler JS, Batchelor TT, Barker FG 2nd. Erlotinib for progressive vestibular schwannoma in neurofibromatosis 2 patients. Otol Neurotol. 2010;31(7):1135–43. https://doi.org/10.1097/MAO.0b013e3181eb328a.
Fong B, Barkhoudarian G, Pezeshkian P, Parsa AT, Gopen Q, Yang I. The molecular biology and novel treatments of vestibular schwannomas. J Neurosurg. 2011;115(5):906–14. https://doi.org/10.3171/2011.6.JNS11131.
Zhao F, Li SW, Zhang S, Li P, Zhao C, Zhao XB, et al. Phase II trial of icotinib in adult patients with neurofibromatosis type 2 and progressive vestibular schwannoma. J Neurosurg. 2022:1–8. https://doi.org/10.3171/2022.9.JNS22699.
Karajannis MA, Legault G, Hagiwara M, Ballas MS, Brown K, Nusbaum AO, et al. Phase II trial of lapatinib in adult and pediatric patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol. 2012;14(9):1163–70. https://doi.org/10.1093/neuonc/nos146.
Ammoun S, Schmid MC, Triner J, Manley P, Hanemann CO. Nilotinib alone or in combination with selumetinib is a drug candidate for neurofibromatosis type 2. Neuro Oncol. 2011;13(7):759–66. https://doi.org/10.1093/neuonc/nor056.
Ammoun S, Evans DG, Hilton DA, Streeter A, Hayward C, Hanemann CO. Phase 0 trial investigating the intratumoural concentration and activity of sorafenib in neurofibromatosis type 2. J Neurol Neurosurg Psychiatry. 2019;90(10):1184–7. https://doi.org/10.1136/jnnp-2018-319713.
Uesaka T, Shono T, Suzuki SO, Nakamizo A, Niiro H, Mizoguchi M, et al. Expression of VEGF and its receptor genes in intracranial schwannomas. J Neurooncol. 2007;83(3):259–66. https://doi.org/10.1007/s11060-007-9336-0.
Plotkin SR, Duda DG, Muzikansky A, Allen J, Blakeley J, Rosser T, et al. Multicenter, prospective, phase II and biomarker study of high-dose bevacizumab as induction therapy in patients with neurofibromatosis type 2 and progressive vestibular schwannoma. J Clin Oncol. 2019;37(35):3446–54. https://doi.org/10.1200/JCO.19.01367.
Mautner VF, Nguyen R, Knecht R, Bokemeyer C. Radiographic regression of vestibular schwannomas induced by bevacizumab treatment: sustain under continuous drug application and rebound after drug discontinuation. Ann Oncol. 2010;21(11):2294–5. https://doi.org/10.1093/annonc/mdq566.
Slusarz KM, Merker VL, Muzikansky A, Francis SA, Plotkin SR. Long-term toxicity of bevacizumab therapy in neurofibromatosis 2 patients. Cancer Chemother Pharmacol. 2014;73(6):1197–204. https://doi.org/10.1007/s00280-014-2456-2.
Phadnis S, Hagiwara M, Yaffe A, Mitchell C, Nicolaides T, Akshintala S, et al. NFB-08 Phase II study of axitinib in patients with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro-Oncology. 2020;22(Supplement_3):iii419-iii. https://doi.org/10.1093/neuonc/noaa222.612.
Walia A, Yang JF, Huang YH, Rosenblatt MI, Chang JH, Azar DT. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim Biophys Acta. 2015;1850(12):2422–38. https://doi.org/10.1016/j.bbagen.2015.09.007.
Packer RJ, Rood BR, Turner DC, Stewart CF, Fisher M, Smith C, et al. Phase I and pharmacokinetic trial of PTC299 in pediatric patients with refractory or recurrent central nervous system tumors: a PBTC study. J Neurooncol. 2015;121(1):217–24. https://doi.org/10.1007/s11060-014-1665-1.
Ammoun S, Ristic N, Matthies C, Hilton DA, Hanemann CO. Targeting ERK1/2 activation and proliferation in human primary schwannoma cells with MEK1/2 inhibitor AZD6244. Neurobiol Dis. 2010;37(1):141–6. https://doi.org/10.1016/j.nbd.2009.09.017.
Zhao Y, Liu P, Zhang N, Chen J, Landegger LD, Wu L, et al. Targeting the cMET pathway augments radiation response without adverse effect on hearing in NF2 schwannoma models. Proc Natl Acad Sci. 2018;115(9):E2077–84. https://doi.org/10.1073/pnas.1719966115.
Lane HA, Wood JM, McSheehy PM, Allegrini PR, Boulay A, Brueggen J, et al. mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res. 2009;15(5):1612–22. https://doi.org/10.1158/1078-0432.Ccr-08-2057.
Santana VM, Sahr N, Tatevossian RG, Jia S, Campagne O, Sykes A, et al. A phase 1 trial of everolimus and bevacizumab in children with recurrent solid tumors. Cancer. 2020;126(8):1749–57. https://doi.org/10.1002/cncr.32722.
Karajannis MA, Legault G, Hagiwara M, Giancotti FG, Filatov A, Derman A, et al. Phase II study of everolimus in children and adults with neurofibromatosis type 2 and progressive vestibular schwannomas. Neuro Oncol. 2014;16(2):292–7. https://doi.org/10.1093/neuonc/not150.
Goutagny S, Raymond E, Esposito-Farese M, Trunet S, Mawrin C, Bernardeschi D, et al. Phase II study of mTORC1 inhibition by everolimus in neurofibromatosis type 2 patients with growing vestibular schwannomas. J Neurooncol. 2015;122(2):313–20. https://doi.org/10.1007/s11060-014-1710-0.
Hawley E, Gehlhausen J, Karchugina S, Chow HY, Araiza-Olivera D, Radu M, et al. PAK1 inhibition reduces tumor size and extends the lifespan of mice in a genetically engineered mouse model of Neurofibromatosis Type 2 (NF2). Hum Mol Genet. 2021;30(17):1607–17. https://doi.org/10.1093/hmg/ddab106.
Lee TX, Packer MD, Huang J, Akhmametyeva EM, Kulp SK, Chen CS, et al. Growth inhibitory and anti-tumour activities of OSU-03012, a novel PDK-1 inhibitor, on vestibular schwannoma and malignant schwannoma cells. Eur J Cancer. 2009;45(9):1709–20. https://doi.org/10.1016/j.ejca.2009.03.013.
Welling DB, Collier KA, Burns SS, Oblinger JL, Shu E, Miles-Markley BA, et al. Early phase clinical studies of AR-42, a histone deacetylase inhibitor, for neurofibromatosis type 2-associated vestibular schwannomas and meningiomas. Laryngoscope Investig Otolaryngol. 2021;6(5):1008–19. https://doi.org/10.1002/lio2.643.
Collier KA, Valencia H, Newton H, Hade EM, Sborov DW, Cavaliere R, et al. A phase 1 trial of the histone deacetylase inhibitor AR-42 in patients with neurofibromatosis type 2-associated tumors and advanced solid malignancies. Cancer Chemother Pharmacol. 2021;87(5):599–611. https://doi.org/10.1007/s00280-020-04229-3.
Angelo LS, Wu JY, Meng F, Sun M, Kopetz S, McCutcheon IE, et al. Combining curcumin (diferuloylmethane) and heat shock protein inhibition for neurofibromatosis 2 treatment: analysis of response and resistance pathways. Mol Cancer Ther. 2011;10(11):2094–103. https://doi.org/10.1158/1535-7163.Mct-11-0243.
Tanaka K, Eskin A, Chareyre F, Jessen WJ, Manent J, Niwa-Kawakita M, et al. Therapeutic potential of HSP90 inhibition for neurofibromatosis type 2. Clin Cancer Res. 2013;19(14):3856–70. https://doi.org/10.1158/1078-0432.Ccr-12-3167.
Muller DN, Heissmeyer V, Dechend R, Hampich F, Park JK, Fiebeler A, et al. Aspirin inhibits NF-kappaB and protects from angiotensin II-induced organ damage. Faseb j. 2001;15(10):1822–4. https://doi.org/10.1096/fj.00-0843fje.
Kandathil CK, Dilwali S, Wu CC, Ibrahimov M, McKenna MJ, Lee H, et al. Aspirin intake correlates with halted growth of sporadic vestibular schwannoma in vivo. Otol Neurotol. 2014;35(2):353–7. https://doi.org/10.1097/mao.0000000000000189.
Dilwali S, Kao SY, Fujita T, Landegger LD, Stankovic KM. Nonsteroidal anti-inflammatory medications are cytostatic against human vestibular schwannomas. Transl Res. 2015;166(1):1–11. https://doi.org/10.1016/j.trsl.2014.12.007.
Ignacio KHD, Espiritu AI, Diestro JDB, Chan KI, Dmytriw AA, Omar AT 2nd. Efficacy of aspirin for sporadic vestibular schwannoma: a meta-analysis. Neurol Sci. 2021;42(12):5101–6. https://doi.org/10.1007/s10072-021-05193-3.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Evan Cumpston and Steven Rhodes declare no conflicts of interest. Charles Yates has served on the Board of Directors for St. Joseph Institute for the Deaf and has served as an expert witness for Aylstock, Witkin, Kreis & Overholtz PLLC.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cumpston, E.C., Rhodes, S.D. & Yates, C.W. Advances in Targeted Therapy for Neurofibromatosis Type 2 (NF2)-Associated Vestibular Schwannomas. Curr Oncol Rep 25, 531–537 (2023). https://doi.org/10.1007/s11912-023-01388-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-023-01388-3