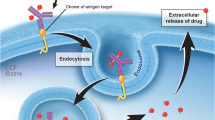
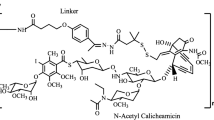
Abstract
Rituximab, a monoclonal antibody (MAb) against CD20, was the first MAb approved by the US Food and Drug Administration (FDA) for treatment of B cell non-Hodgkin lymphoma (B-NHL). Conjugating toxins to MAb was a technical challenge; however, with improvements in linker technology, immunoconjugates were constructed and revolutionized cancer treatment. Gemtuzumab ozogamicin was the first antibody drug conjugate (ADC) approved by the FDA. Because of the success of brentuximab vedotin and ado-trastuzumab emtansine in treating Hodgkin lymphoma (HL) and HER2-positive breast cancer, respectively, newer ADCs are being investigated. Brentuximab vedotin is approved for both HL and anaplastic large cell lymphoma. Newer ADCs, such as polatuzumab vedotin (targeting CD79b), pinatuzumab vedotin (targeting CD22), inotuzumab ozogamicin (targeting CD19), SAR3419 (targeting CD19), IMGN529 (targeting CD37), and SGN-CD19A (targeting CD19), have shown promising preclinical and early clinical activity. These findings will change the landscape of B-NHL treatment away from age-old “CHOP”-based chemotherapies.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Skipper HE. Laboratory models: some historical perspective. Cancer Treat Rep. 1986;70(1):3–7.
Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012;30(7):631–7. This is a very good review detailing the development of BV.
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7.
Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6(5):343–57.
McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825–33.
http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm109106.htm. (Ed.^(Eds).
Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47(2):115–23.
Bouchard H, Viskov C, Garcia-Echeverria C. Antibody-drug conjugates—a new wave of cancer drugs. Bioorg Med Chem Lett. 2014;24(23):5357–63. This is a good review of the development of antibody–drug conjugates for cancer treatment.
Palanca-Wessels MC, Press OW. Advances in the treatment of hematologic malignancies using immunoconjugates. Blood. 2014;123(15):2293–301. This review article describes the current development of ADCs for treating hematologic malignancies.
Dosio F, Stella B, Cerioni S, Gastaldi D, Arpicco S. Advances in anticancer antibody-drug conjugates and immunotoxins. Recent Pat Anticancer Drug Discov. 2014;9(1):35–65.
Trail PA. Antibody drug conjugates as cancer therapeutics. Antibodies. 2013;2(1):113–29.
Tolcher AW, Sugarman S, Gelmon KA, et al. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J Clin Oncol. 1999;17(2):478–84.
Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19(13):3244–54.
Bross PF, Beitz J, Chen G, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7(6):1490–6.
Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–60.
Vaklavas C, Forero-Torres A. Safety and efficacy of brentuximab vedotin in patients with Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Ther Adv Hematol. 2012;3(4):209–25. This article compiles information on BV development and clinical trials.
Francisco JA, Cerveny CG, Meyer DL, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102(4):1458–65.
Sutherland MS, Sanderson RJ, Gordon KA, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem. 2006;281(15):10540–7.
Oflazoglu E, Kissler KM, Sievers EL, Grewal IS, Gerber HP. Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br J Haematol. 2008;142(1):69–73.
Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–21.
Fanale MA, Forero-Torres A, Rosenblatt JD, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res. 2012;18(1):248–55.
Younes A, Connors JM, Park SI, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed Hodgkin’s lymphoma: a phase 1, open-label, dose-escalation study. Lancet Oncol. 2013;14(13):1348–56.
Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137–43.
Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–9.
Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–6.
Dornan D, Bennett F, Chen Y, et al. Therapeutic potential of an anti-CD79b antibody-drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood. 2009;114(13):2721–9.
Polson AG, Yu SF, Elkins K, et al. Antibody-drug conjugates targeted to CD79 for the treatment of non-Hodgkin lymphoma. Blood. 2007;110(2):616–23.
Junutula JR, Raab H, Clark S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925–32.
Palanca-Wessels MC, Flinn IW, Sehn LH, Patel M, Sangha R, Czuczman MS, et al. A Phase I study of the anti-CD79b antibody-drug conjugate (ADC) DCDS4501A targeting CD79b in relapsed or refractory B-Cell non-Hodgkin’s lymphoma (NHL). (Ed.^(Eds) (American Society of Hematology, 54th Annual meeting).
Rossi EA, Goldenberg DM, Michel R, Rossi DL, Wallace DJ, Chang CH. Trogocytosis of multiple B-cell surface markers by CD22 targeting with epratuzumab. Blood. 2013;122(17):3020–9.
Polson AG, Williams M, Gray AM, et al. Anti-CD22-MCC-DM1: an antibody-drug conjugate with a stable linker for the treatment of non-Hodgkin’s lymphoma. Leukemia. 2010;24(9):1566–73.
Morschhauser IF, Advani RH, Sehn LH, Kolibaba KS, Press OW, Salles GA, et al. Preliminary results of a phase II randomized study (ROMULUS) of polatuzumab vedotin (PoV) or pinatuzumab vedotin (PiV) plus rituximab (RTX) in patients (Pts) with relapsed/refractory (R/R) non-Hodgkin lymphoma (NHL). (Ed.^(Eds) (American Society of Clinical Oncology (ASCO), 2014 meeting. Abstract No:8519, 2014).
DiJoseph JF, Armellino DC, Boghaert ER, et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103(5):1807–14.
Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21(11):2240–5.
DiJoseph JF, Goad ME, Dougher MM, et al. Potent and specific antitumor efficacy of CMC-544, a CD22-targeted immunoconjugate of calicheamicin, against systemically disseminated B-cell lymphoma. Clin Cancer Res. 2004;10(24):8620–9.
Ogura M, Hatake K, Ando K, et al. Phase I study of anti-CD22 immunoconjugate inotuzumab ozogamicin plus rituximab in relapsed/refractory B-cell non-Hodgkin lymphoma. Cancer Sci. 2012;103(5):933–8.
Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13(4):403–11.
Fayad L, Offner F, Smith MR, et al. Safety and clinical activity of a combination therapy comprising two antibody-based targeting agents for the treatment of non-Hodgkin lymphoma: results of a phase I/II study evaluating the immunoconjugate inotuzumab ozogamicin with rituximab. J Clin Oncol. 2013;31(5):573–83.
Wagner-Johnston ND, Goy A, Rodriguez MA et al. A Phase 2 study of inotuzumab ozogamicin and rituximab, followed by autologous stem cell transplantation in with relapsed/refractory diffuse large B-Cell lymphoma. Leuk Lymphoma, 1–27 (2015).
Blanc V, Bousseau A, Caron A, Carrez C, Lutz RJ, Lambert JM. SAR3419: an anti-CD19-Maytansinoid Immunoconjugate for the treatment of B-cell malignancies. Clin Cancer Res. 2011;17(20):6448–58.
Lutz RJ, Zuany-Amorim C, Vrignaud P et al. Preclinical evaluation of SAR3419 (huB4-DM4), an anti-CD19-maytansinoid immunoconjugate, for the treatment of B cell lymphomas. In: AACR Meeting Abstracts. (Ed.^(Eds) (2006) 877.
Al-Katib AM, Aboukameel A, Mohammad R, Bissery MC, Zuany-Amorim C. Superior antitumor activity of SAR3419 to rituximab in xenograft models for non-Hodgkin’s lymphoma. Clin Cancer Res. 2009;15(12):4038–45.
Younes A, Kim S, Romaguera J, et al. Phase I multidose-escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous infusion every 3 weeks to patients with relapsed/refractory B-cell lymphoma. J Clin Oncol. 2012;30(22):2776–82.
Ribrag V, Dupuis J, Tilly H, et al. A dose-escalation study of SAR3419, an anti-CD19 antibody maytansinoid conjugate, administered by intravenous infusion once weekly in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2014;20(1):213–20.
Press OW, Eary JF, Badger CC, et al. Treatment of refractory non-Hodgkin’s lymphoma with radiolabeled MB-1 (anti-CD37) antibody. J Clin Oncol. 1989;7(8):1027–38.
Zhao X, Lapalombella R, Joshi T, et al. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood. 2007;110(7):2569–77.
Deckert J, Park PU, Chicklas S, et al. A novel anti-CD37 antibody-drug conjugate with multiple anti-tumor mechanisms for the treatment of B-cell malignancies. Blood. 2013;122(20):3500–10.
Forero-Torres A, Moskowitz C, Advani RH, Shah BD, Kostic A, Albertson TM, et al. Interim analysis of a phase 1, open-label, dose-escalation study of SGN-CD19A in patients with relapsed or refractory B-lineage non-Hodgkin lymphoma (NHL). (Ed.^(Eds) (American Society of Clinical Oncology, annual meeting 2014, 2014).
Uma Borate M, Fathi AT, Shah BD, DeAngelo DJ, Silverman LB, Cooper TM, et al. A first-in-human phase 1 study of the antibody-drug conjugate SGN-CD19A in relapsed or refractory b-lineage acute leukemia and highly aggressive lymphoma. (Ed.^(Eds) (American Society of Hematology, Annual meeting 2013. Abstract No: 1437, 2013).
Coiffier B, Ribrag V, Dupuis J, et al. Phase I/II study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered weekly to patients (pts) with relapsed/refractory B-cell non-Hodgkin lymphoma (NHL). J Clin Oncol. 2011;29:508s.
Compliance with Ethics Guidelines
Conflict of Interest
Amitkumar Mehta has received research support through grants from Seattle Genetics, MedImmune, and Genentech.
Andres Forero-Torres has received research support through grants from Seattle Genetics, MedImmune, and Genentech.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Lymphomas
Rights and permissions
About this article
Cite this article
Mehta, A., Forero-Torres, A. Development and Integration of Antibody–Drug Conjugate in Non-Hodgkin Lymphoma. Curr Oncol Rep 17, 41 (2015). https://doi.org/10.1007/s11912-015-0466-9
Published:
DOI: https://doi.org/10.1007/s11912-015-0466-9