Abstract
Purpose of Review
The purpose of this article is to review the current approaches using neuroimaging techniques to expand eligibility for intravenous thrombolytic therapy in acute ischemic stroke patients with stroke of unknown symptom onset.
Recent Findings
In recent years, several randomized, placebo-controlled trials have shown neuroimaging-guided approaches to be feasible in determining eligibility for alteplase beyond 4.5 h from last known well, and efficacious for reducing disability. DWI-FLAIR mismatch on MRI is an effective tool to identify stroke lesions less than 4.5 h in onset in patients with stroke of unknown symptom onset. Additionally, an automated perfusion-based approach, assessing for a disproportionate amount of salvageable tissue, is effective in identifying patients likely to benefit from late window alteplase treatment.
Summary
In patients with stroke of unknown symptom onset, an individualized approach using neuroimaging to determine time of stroke onset or presence of salvageable brain tissue is feasible in the acute setting and associated with improved long-term outcomes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA stroke study group. N Engl J Med. 1995;333:1581–7.
Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29.
Adeoye O, Hornung R, Khatri P, Kleindorfer D. Recombinant tissue-type plasminogen activator use for ischemic stroke in the United States: a doubling of treatment rates over the course of 5 years. Stroke. 2011;42:1952–5.
California Acute Stroke Pilot Registry I. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64:654–9.
Maas MB, Singhal AB. Unwitnessed stroke: impact of different onset times on eligibility into stroke trials. J Stroke Cerebrovasc Dis. 2013;22:241–3.
Kim YJ, Kim BJ, Kwon SU, Kim JS, Kang DW. Unclear-onset stroke: Daytime-unwitnessed stroke vs wake-up stroke. Int J Stroke. 2016;11:212–20.
Fink JN, Kumar S, Horkan C, Linfante I, Selim MH, Caplan LR, et al. The stroke patient who woke up: clinical and radiological features, including diffusion and perfusion MRI. Stroke. 2002;33:988–93.
Marler JR, Price TR, Clark GL, Muller JE, Robertson T, Mohr JP, et al. Morning increase in onset of ischemic stroke. Stroke. 1989;20:473–6.
Mackey J, Kleindorfer D, Sucharew H, Moomaw CJ, Kissela BM, Alwell K, et al. Population-based study of wake-up strokes. Neurology. 2011;76:1662–7.
Etherton MR, Barreto AD, Schwamm LH, Wu O. Neuroimaging paradigms to identify patients for reperfusion therapy in stroke of unknown onset. Front Neurol. 2018;9:327.
Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European cooperative acute stroke study (ECASS). JAMA. 1995;274:1017–25.
Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian acute stroke study investigators. Lancet. 1998;352:1245–51.
Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282:2019–26.
Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363:768–74.
Powers WJ, Rabinstein AA, Ackerson T, et al. Update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;2019:STR0000000000000211.
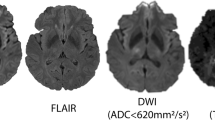
Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol. 2011;10:978–86.
Thomalla G, Rossbach P, Rosenkranz M, Siemonsen S, Krützelmann A, Fiehler J, et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Ann Neurol. 2009;65:724–32.
Ebinger M, Galinovic I, Rozanski M, Brunecker P, Endres M, Fiebach JB. Fluid-attenuated inversion recovery evolution within 12 hours from stroke onset: a reliable tissue clock? Stroke. 2010;41:250–5.
Aoki J, Kimura K, Iguchi Y, Shibazaki K, Sakai K, Iwanaga T. FLAIR can estimate the onset time in acute ischemic stroke patients. J Neurol Sci. 2010;293:39–44.
Petkova M, Rodrigo S, Lamy C, Oppenheim G, Touzé E, Mas JL, et al. MR imaging helps predict time from symptom onset in patients with acute stroke: implications for patients with unknown onset time. Radiology. 2010;257:782–92.
•• Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611–22 This Phase 3 randomized, placebo-controlled trial showed that DWI-FLAIR mismatch was feasible in guiding alteplase decision-making in stroke of uknown symptom onset beyond 4.5 hours of LKW and associated with reduced disability at 90-days.
Schwamm LH, Wu O, Song SS, Latour LL, Ford AL, Hsia AW, et al. Intravenous thrombolysis in unwitnessed stroke onset: MR WITNESS trial results. Ann Neurol. 2018;83:980–93.
Koga M, Toyoda K, Kimura K, Yamamoto H, Sasaki M, Hamasaki T, et al. THrombolysis for Acute Wake-up and unclear-onset Strokes with alteplase at 0.6 mg/kg (THAWS) Trial. Int J Stroke. 2014;9:1117–24.
Schwamm LH, Wu O, Song SS, et al. IV Alteplase in MR-selected Patients with Stroke of Unknown Onset is Safe and Feasible: Results of the Multicenter MR WITNESS Trial (NCT01282242). Int Stroke Conf. 2016. Los Angeles, CA.
Rocha M, Jovin TG. Fast versus slow Progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke. 2017;48:2621–7.
Campbell BC, Purushotham A, Christensen S, et al. The infarct core is well represented by the acute diffusion lesion: sustained reversal is infrequent. J Cereb Blood Flow Metab. 2012;32:50–6.
Wintermark M, Albers GW, Broderick JP, Demchuk AM, Fiebach JB, Fiehler J, et al. Acute stroke imaging research roadmap II. Stroke. 2013;44:2628–39.
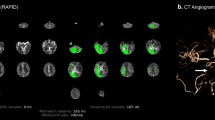
Campbell BC, Christensen S, Levi CR, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–40.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18.
Sorensen AG, Buonanno FS, Gonzalez RG, Schwamm LH, Lev MH, Huang-Hellinger FR, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology. 1996;199:391–401.
Sorensen AG, Copen WA, Ostergaard L, et al. Hyperacute stroke: simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology. 1999;210:519–27.
Baird AE, Warach S. Magnetic resonance imaging of acute stroke. J Cereb Blood Flow Metab. 1998;18:583–609.
Warach S, Dashe JF, Edelman RR. Clinical outcome in ischemic stroke predicted by early diffusion-weighted and perfusion magnetic resonance imaging: a preliminary analysis. J Cereb Blood Flow Metab. 1996;16:53–9.
Shih LC, Saver JL, Alger JR, Starkman S, Leary MC, Vinuela F, et al. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke. 2003;34:1425–30.
Wu O, Koroshetz WJ, Ostergaard L, et al. Predicting tissue outcome in acute human cerebral ischemia using combined diffusion- and perfusion-weighted MR imaging. Stroke. 2001;32:933–42.
Wu O, Christensen S, Hjort N, et al. Characterizing physiological heterogeneity of infarction risk in acute human ischaemic stroke using MRI. Brain. 2006;129:2384–93.
Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar imaging thrombolytic evaluation trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309.
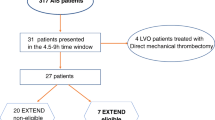
•• Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380:1795–803 This Phase 3 randomized, placebo-controlled trial showed that a perfusion-based imaging approach to identify target mismatch in acute ischemic stroke patients 4.5–9 hours from LKW was feasible for alteplase decision-making and resulted in reduced long-term disability.
Ma H, Parsons MW, Christensen S, Campbell BCV, Churilov L, Connelly A, et al. A multicentre, randomized, double-blinded, placebo-controlled phase III study to investigate EXtending the time for thrombolysis in emergency neurological deficits (EXTEND). Int J Stroke. 2012;7:74–80.
Campbell BCV, Ma H, Ringleb PA, Parsons MW, Churilov L, Bendszus M, et al. Extending thrombolysis to 4.5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet. 2019;394:139–47.
Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2009;8:141–50.
Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366:1099–107.
Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2017.
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18.
Wu O, Schwamm LH, Sorensen AG. Imaging stroke patients with unclear onset times. Neuroimaging Clin N Am. 2011;21:327–44 xi.
Chaturvedi S, Adams HP Jr, Woolson RF. Circadian variation in ischemic stroke subtypes. Stroke. 1999;30:1792–5.
Serena J, Davalos A, Segura T, Mostacero E, Castillo J. Stroke on awakening: looking for a more rational management. Cerebrovasc Dis. 2003;16:128–33.
Lago A, Geffner D, Tembl J, Landete L, Valero C, Baquero M. Circadian variation in acute ischemic stroke: a hospital-based study. Stroke. 1998;29:1873–5.
Argentino C, Toni D, Rasura M, Violi F, Sacchetti ML, Allegretta A, et al. Circadian variation in the frequency of ischemic stroke. Stroke. 1990;21:387–9.
Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29:992–6.
Todo K, Moriwaki H, Saito K, Tanaka M, Oe H, Naritomi H. Early CT findings in unknown-onset and wake-up strokes. Cerebrovasc Dis. 2006;21:367–71.
Denny MC, Boehme AK, Dorsey AM, et al. Wake-up strokes are similar to known-onset morning strokes in severity and outcome. J Neurol Neurol Disord. 2014;1.
Moradiya Y, Janjua N. Presentation and outcomes of "wake-up strokes" in a large randomized stroke trial: analysis of data from the international stroke trial. J Stroke Cerebrovasc Dis. 2013;22:e286–92.
Barreto AD, Martin-Schild S, Hallevi H, Morales MM, Abraham AT, Gonzales NR, et al. Thrombolytic therapy for patients who wake-up with stroke. Stroke. 2009;40:827–32.
Manawadu D, Bodla S, Jarosz J, Keep J, Kalra L. A case-controlled comparison of thrombolysis outcomes between wake-up and known time of onset ischemic stroke patients. Stroke. 2013;44:2226–31.
Barreto AD, Fanale CV, Alexandrov AV, Gaffney KC, Vahidy FS, Nguyen CB, et al. Prospective, open-label safety study of intravenous recombinant tissue plasminogen activator in wake-up stroke. Ann Neurol. 2016;80:211–8.
ClinicalTrials.gov. Safety of Intravenous Thrombolytics in Stroke on Awakening [online].
Urrutia VC, Faigle R, Zeiler SR, Marsh EB, Bahouth M, Cerdan Trevino M, et al. Safety of intravenous alteplase within 4.5 hours for patients awakening with stroke symptoms. PLoS One. 2018;13:e0197714.
Tenecteplase in Wake-up Ischaemic Stroke Trial [online].
Disclosures
Drs. Etherton and Gadhia report no disclosures. Dr. Schwamm reports the following relationships relevant to research grants or companies that manufacture products for telemedicine, thrombolysis or thrombectomy: scientific consultant regarding trial design and conduct to Genentech (late window thrombolysis); user interface design and usability to LifeImage (and holds < 1% stock options in this privately held company); stroke systems of care to the Massachusetts Department of Public Health; member of a Data Safety Monitoring Board (DSMB) for Penumbra (Separator 3D NCT01584609, last payment 2016; MIND NCT03342664, CURRENT); Diffusion Pharma PHAST-TSC NCT03763929, CURRENT); National PI or member of National Steering Committee for Medtronic (Victory AF NCT01693120, last payment 2015; Stroke AF NCT02700945, CURRENT); PI, late window thrombolysis trial, NINDS (P50NS051343, MR WITNESS NCT01282242; last payment 2017 and alteplase provided free of charge to Massachusetts General Hospital as well as supplemental per-patient payments to participating sites last payment 2017); PI, StrokeNet Network NINDS (New England Regional Coordinating Center U24NS107243, CURENT); Co-I, The Impact of Telestroke on Patterns of Care and Long-Term Outcomes, NINDS (R01NS111952; CURRENT); Co-I, REACH-PC, PCORI (NCT03375489; CURRENT); Member of steering committee, Genentech (TIMELESS NCT03785678, CURRENT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Stroke
Rights and permissions
About this article
Cite this article
Etherton, M.R., Gadhia, R.R. & Schwamm, L.H. Thrombolysis beyond 4.5 h in Acute Ischemic Stroke. Curr Neurol Neurosci Rep 20, 35 (2020). https://doi.org/10.1007/s11910-020-01055-1
Published:
DOI: https://doi.org/10.1007/s11910-020-01055-1