Abstract
Purpose of the Review
To discuss the diagnostic approach to patients with septic encephalopathy as well as the need for specific neuro-monitoring and the perspectives on future therapeutic approaches in this setting.
Recent Findings
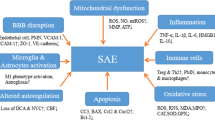
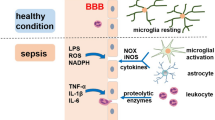
Most of data-concern experimental studies evaluating the pathophysiology of septic encephalopathy. A combination of neurodegenerative pathways with neurovascular injury is the cornerstone for the development of such complication and the long-term neurological sequelae among survivors.
Summary
Septic encephalopathy is a common complication in septic patients. Clinical presentation may range from mild confusion and disorientation to convulsions and deep coma. The diagnosis of septic encephalopathy is made difficult by the lack of any specific clinical and non-clinical feature, in particular among sedated patients in whom neurological examination is unreliable. In spite of the high mortality rate associated with this condition, there is no prophylactic or targeted therapy to reduce or minimize brain damage in septic patients and clinical management is limited to the treatment of the underlying infection.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Vandijck DM, Reynvoet E, Blot SI, Vandecasteele E, Hoste EA. Severe infection, sepsis and acute kidney injury. Acta Clin Belg Suppl. 2007;2:332–6.
Thursky K, Lingaratnam S, Jayarajan J, Haeusler GM, Teh B, Tew M, et al. Implementation of a whole of hospital sepsis clinical pathway in a cancer hospital: impact on sepsis management, outcomes and costs. BMJ Open Qual. 2018;7(3):e000355.
•• Coopersmith CM, De Backer D, Deutschman CS, Ferrer R, Lat I, Machado FR, Martin GS, Martin-Loeches I, Nunnally ME, Antonelli M, Evans LE, Hellman J, Jog S, Kesecioglu J, Levy MM, Rhodes A. Surviving sepsis campaign: research priorities for sepsis and septic shock. Intensive Care Med 2018. Recent review article summarizing the priorities for research in sepsis.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55.
•• Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10 Recent definitions of sepsis according to an international panel of experts and analysis of large US databases.
Eidelman LA, Putterman D, Putterman C, Sprung CL. The spectrum of septic encephalopathy. Definitions, etiologies, and mortalities. JAMA. 1996;275(6):470–3.
Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):762–74.
Wilson JX, Young GB. Sepsis-associated encephalopathy: evolving concepts. Can J Neurol Sci. 2003;30:98–105.
Young GB, Bolton CF, Austin TW, Archibald YM, Gonder J, Wells GA. The encephalopathy associated with sepsis illness. Clin Invest Med. 1990;13:297–304.
Davies NWS, Sharief MK, Howard RS. Infection-associated encephalopathies - their investigation, diagnosis and treatment. J Neurol. 2006;253:833–45.
Sharshar T, Citerio G, Andrews PJ, Chieregato A, Latronico N, Menon DK, et al. Neurological examination of critically ill patients: a pragmatic approach. Report of an ESICM expert panel. Intensive Care Med. 2014;40(4):484–95.
Siami S, Annane D, Sharshar T. The encephalopathy in sepsis. Crit Care Clin. 2008;24:67–82.
Wuerfel E, Infante-Duarte C, Glumm R, Wuerfel JT. Gadofluorine M-enhanced MRI shows involvement of circumventricular organs in neuroinflammation. J Neuroinflammation. 2010;7:70.
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62.
Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, et al. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet. 2003;362:1799–805.
Alexander JJ, Jacob A, Cunningham P, Hensley L, Quigg RJ. TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem Int. 2008;52:447–56.
Chong DL, Sriskandan S. Pro-inflammatory mechanisms in sepsis. Contrib Microbiol. 2011;17:86–107.
Rorato R, Menezes AM, Giusti-Paiva A, de Castro M, Antunes-Rodrigues J, Elias LL. Prostaglandin mediates endotoxaemia-induced hypophagia by activation of pro-opiomelanocortin and corticotrophin-releasing factor neurons in rats. Exp Physiol. 2009;94(3):371–9.
• Rump K, Adamzik M. Function of aquaporins in sepsis: a systematic review. Cell Biosci. 2018;8:10 Interesting and completed review on the role of aquaporins in sepsis, including septic encephalopathy.
Takatani Y, Ono K, Suzuki H, Inaba M, Sawada M, Matsuda N. Inducible nitric oxide synthase during the late phase of sepsis is associated with hypothermia and immune cell migration. Lab Investig. 2018;98(5):629–39.
Ning Q, Liu Z, Wang X, Zhang R, Zhang J, Yang M, et al. Neurodegenerative changes and neuroapoptosis induced by systemic lipopolysaccharide administration are reversed by dexmedetomidine treatment in mice. Neurol Res. 2017;39(4):357–66.
Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T, Klockgether T, et al. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol. 2007;204:733–40.
Kadoi Y, Saito S. An alteration in the gamma-aminobutyric acid receptor system in experimentally induced septic shock in rats. Crit Care Med. 1996;24:298–305.
• Zhai Q, Lai D, Cui P, Zhou R, Chen Q, Hou J, et al. Selective activation of basal forebrain cholinergic neurons attenuates polymicrobial sepsis-induced inflammation via the cholinergic anti-inflammatory pathway. Crit Care Med. 2017;45(10):e1075–82 Experimental studies showing the involvement of cholinergic pathways in the immunomodulation within the cerebral tissue during sepsis.
van Eijk MM, Roes KC, Honing ML, Kuiper MA, Karakus A, van der Jagt M, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376(9755):1829–37.
Freund HR, Muggia-Sullam M, LaFrance R, Holroyde J, Fischer JE. Regional brain amino acid and neurotransmitter derangements during abdominal sepsis and septic encephalopathy in the rat. The effect of amino acid infusions. Arch Surg. 1986;121:209–16.
•• Dahl RH, Berg RMG, Taudorf S, Bailey DM, Lundby C, Larsen FS, et al. A reassessment of the blood-brain barrier transport of large neutral amino acids during acute systemic inflammation in humans. Clin Physiol Funct Imaging. 2018;38(4):656–62 Clinical study describing the role of the amino acids metabolism during acute inflammatory status according to the blood-brain barrier integrity.
Berg RM, Taudorf S, Bailey DM, Lundby C, Larsen FS, Pedersen BK, et al. Cerebral net exchange of large neutral amino acids after lipopolysaccharide infusion in healthy humans. Crit Care. 2010;14(1):R16.
Zhao YZ, Gao ZY, Ma LQ, Zhuang YY, Guan FL. Research on biogenesis of mitochondria in astrocytes in sepsis-associated encephalopathy models. Eur Rev Med Pharmacol Sci. 2017;21(17):3924–34.
Zhan RZ, Fujiwara N, Shimoji K. Regionally different elevation of intracellular free calcium in hippocampus of septic rat brain. Shock. 1996;6:293–7.
• Dhaya I, Griton M, Raffard G, Amri M, Hiba B, Konsman JP. Bacterial lipopolysaccharide-induced systemic inflammation alters perfusion of white matter-rich regions without altering flow in brain-irrigating arteries: relationship to blood-brain barrier breakdown? J Neuroimmunol. 2018;314:67–80 Experimental study suggesting a role for impairment of the blood-brain barrier in the alteration of white matter perfusion, in relationship with microvascular dysfunction.
Rodrigues SF, Granger DN. Blood cells and endothelial barrier function. Tissue Barriers. 2015;3(1–2):e978720.
He H, Geng T, Chen P, Wang M, Hu J, Kang L, et al. NK cells promote neutrophil recruitment in the brain during sepsis-induced neuroinflammation. Sci Rep. 2016;6:27711.
Taccone FS, Su F, Pierrakos C, He X, James S, Dewitte O, et al. Cerebral microcirculation is impaired during sepsis: an experimental study. Crit Care. 2010;14(4):R140.
Taccone FS, Su F, De Deyne C, Abdellhai A, Pierrakos C, He X, et al. Sepsis is associated with altered cerebral microcirculation and tissue hypoxia in experimental peritonitis. Crit Care Med. 2014;42(2):e114–22.
Schramm P, Klein KU, Falkenberg L, Berres M, Closhen D, Werhahn KJ, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care. 2012;16(5):R181.
Taccone FS, Castanares-Zapatero D, Peres-Bota D, Vincent JL, Berre’ J, Melot C. Cerebral autoregulation is influenced by carbon dioxide levels in patients with septic shock. Neurocrit Care. 2010;12(1):35–42.
Taccone FS, Scolletta S, Franchi F, Donadello K, Oddo M. Brain perfusion in sepsis. Curr Vasc Pharmacol. 2013;11(2):170–86.
Ebersoldt M, Sharshar T, Annane D. Sepsis-associated delirium. Intensive Care Med. 2007;33:941–50.
Leon A, Lepousé C, Floch T, Graftieaux JP. Brain injury during severe sepsis. Ann Fr Anesth Reanim. 2006;25:863–7.
Peterson JF, Pun BT, Dittus RS, Thomason JWW, Jackson JC, Shintani AK, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–84.
•• Barichello T, Sayana P, Giridharan VV, Arumanayagam AS, Narendran B, Della Giustina A, Petronilho F, Quevedo J, Dal-Pizzol F. Long-term cognitive outcomes after sepsis: a translational systematic review. Mol Neurobiol 2018. Recent systematic review dealing with the occurrence of long-term cognitive dysfunction in both experimental and clinical studies.
Semmler A, Widmann CN, Okulla T, Urbach H, Kaiser M, Widman G, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry. 2013;84:62–70.
Gunther ML, Morandi A, Krauskopf E, Pandharipande P, Girard TD, Jackson JC, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study. Crit Care Med. 2012;40:2022–32.
Wang LM, Wu Q, Kirk RA, Horn KP, Ebada Salem AH, Hoffman JM, et al. Lipopolysaccharide endotoxemia induces amyloid-β and p-tau formation in the rat brain. Am J Nucl Med Mol Imaging. 2018;8(2):86–99.
Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–10.
Riker RR, Fugate JE, Participants in the International Multi-disciplinary Consensus Conference on Multimodality Monitoring. Clinical monitoring scales in acute brain injury: assessment of coma, pain, agitation, and delirium. Neurocrit Care. 2014;21(Suppl 2):S27–37.
Venkatesh B, Scott P, Ziegenfuss M. Cerebrospinal fluid in critical illness. Crit Care Resusc. 2000;2(1):42–54.
Hosokawa K, Gaspard N, Su F, Oddo M, Vincent JL, Taccone FS. Clinical neurophysiological assessment of sepsis-associated brain dysfunction: a systematic review. Crit Care. 2014;18:674.
Oddo M, Carrera E, Claassen J, SA M, LJ H. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051–6.
•• Reznik ME, Merkler AE, Mahta A, Murthy SB, Claassen J, Kamel H. Long-term risk of seizures in adult survivors of sepsis. Neurology. 2017;89(14):1476–82 Large cohort study showing that survivors of sepsis face a significantly higher long-term risk of seizures than other patients.
• Admiraal MM, Gilmore EJ, Van Putten MJAM, Zaveri HP, Hirsch LJ, Gaspard N. Disruption of brain-heart coupling in sepsis. J Clin Neurophysiol. 2017;34(5):413–20 Physiologic study associating the loss of EEG reactivity to the dysfunction of the autonomic system, which was evaluated by the assessment of heart rate varibility.
Piazza O, Cotena S, De Robertis E, Caranci F, Tufano R. Sepsis associated encephalopathy studied by MRI and cerebral spinal fluid S100B measurement. Neurochem Res. 2009;34:1289–92.
• Anderson BJ, Reilly JP, Shashaty MGS, Palakshappa JA, Wysoczanski A, Dunn TG, et al. Admission plasma levels of the neuronal injury marker neuron-specific enolase are associated with mortality and delirium in sepsis. J Crit Care. 2016;36:18–23 Observational study showing a potential prognostic role for biomarkers of brain injury in septic patient.
Pfister D, Siegemund M, Dell-Kuster S, Smielewski P, Rüegg S, Strebel SP, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12:R63.
Nguyen DN, Spapen H, Su F, Schiettecatte J, Shi L, Hachimi-Idrissi S, et al. Elevated serum levels of S-100β protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med. 2006;34:1967–74.
Polito A, Eischwald F, Maho ALL, Polito A, Azabou E, Annane D, et al. Pattern of brain injury in the acute setting of human septic shock. Crit Care. 2013;17:R204.
Finelli PF, Uphoff DF. Magnetic resonance imaging abnormalities with septic encephalopathy. J Neurol Neurosurg Psychiatry. 2004;75:1189–91.
Sharshar T, Carlier R, Bernard F, Guidoux C, Brouland JP, Nardi O, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33:798–806.
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–62.
Bleck TP, Smith MC, Pierre-Louis SJ, Jares JJ, Murray J, Hansen CA. Neurologic complications of critical medical illness. Crit Care Med. 1993;21:98–103.
Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF, Sheagren JN, et al. Impact of encephalopathy on mortality in sepsis syndrome. Crit Care Med. 1990;18:474–9.
•• Sonneville R, de Montmollin E, Poujade J, Garrouste-Orgeas M, Souweine B, Darmon M, et al. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med. 2017;43(8):1075–84 Recent large cohort study investigating the predictors of brain dysfunction in septic patients.
Oddo M, Taccone FS. How to monitor the brain in septic patients? Minerva Anestesiol. 2015;81:776–88.
Vasko A, Siro P, Laszlo I, Szatmari S, Molnar L, Fulesdi B, et al. Assessment of cerebral tissue oxygen saturation in septic patients during acetazolamide provocation - a near infrared spectroscopy study. Acta Physiol Hung. 2014;101:32–9.
Donnelly J, Aries MJ, Czosnyka M. Further understanding of cerebral autoregulation at the bedside: possible implications for future therapy. Expert Rev Neurother. 2015;15:169–85.
Minati L, Kress IU, Visani E, Medford N, Critchley HD. Intra- and extra-cranial effects of transient blood pressure changes on brain near-infrared spectroscopy (NIRS) measurements. J Neurosci Methods. 2011;197(2):283–8.
Robba C, Cardim D, Sekhon M, Budohoski K, Czosnyka M. Transcranial doppler: a stethoscope for the brain-neurocritical care use. J Neurosci Res. 2018;96(4):720–30.
Pierrakos C, Antoine A, Velissaris D, Michaux I, Bulpa P, Evrard P, et al. Transcranial doppler assessment of cerebral perfusion in critically ill septic patients: a pilot study. Ann Intensive Care. 2013;3:28.
Foreman B, Claassen J, Khaled KA, Jirsch J, Alschuler DM, JohnWittman J, et al. Generalized periodic discharges in the critically ill: a case-control study of 200 patients. Neurology. 2012;79:1951–60.
•• Andresen JM, Girard TD, Pandharipande PP, Davidson MA, Ely EW, Watson PL. Burst suppression on processed electroencephalography as a predictor of postcoma delirium in mechanically ventilated ICU patients. Crit Care Med. 2014;42(10):2244–51 Observational study suggesting an association between the depth of anesthesia, which was assessed by the burst suppression rate on EEG, and the occurrence of delirium in ventilated critically ill patients.
Luitse MJ, van Asch CJ, Klijn CJ. Deep coma and diffuse white matter abnormalities caused by sepsis-associated encephalopathy. Lancet. 2013;381(9884):2222.
•• Beumier M, Casu GS, Hites M, Wolff F, Cotton F, Vincent JL, et al. Elevated Beta-lactam concentrations are associ- ated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015;81:497–506 Large cohort study suggesting a role for elevated through β-lactam concentrations and the occurrence of neurological deterioration in septic patients.
Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA Jr, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41.
Polito A, Brouland JP, Porcher R, Sonneville R, Siami S, Stevens RD, et al. Hyperglycaemia and apoptosis of micro-glial cells in human septic shock. Crit Care. 2011;15:R131.
Bagshaw SM, Peets AD, Hameed M, Boiteau PJ, Laupland KB, Doig CJ. Dialysis disequilibrium syndrome: brain death following hemodialysis for metabolic acidosis and acute renal failure–a case report. BMC Nephrol. 2004;5:9.
Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, Shintani AK, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38.
Zhang X, Yan F, Feng J, Qian H, Cheng Z, Yang Q, et al. Dexmedetomidine inhibits inflammatory reaction in the hippocampus of septic rats by suppressing NF-κB pathway. PLoS One. 2018;13(5):e0196897.
Spapen H, Nguyen DN, Troubleyn J, Huyghens L, Schiettecatte J. Drotrecogin alfa (activated) may attenuate severe sepsis-associated encephalopathy in clinical septic shock. Crit Care. 2010;14:R54. https://doi.org/10.1186/cc8947.
Reis PA, Alexandre PCB, D'Avila JC, Siqueira LD, Antunes B, Estato V, et al. Statins prevent cognitive impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction. Brain Behav Immun. 2017;60:293–303.
Esen F, Senturk E, Ozcan PE, Ahishali B, Arican N, Orhan N, et al. Intravenous immunoglobulins prevent the breakdown of the blood-brain barrier in experimentally induced sepsis. Crit Care Med. 2012;40(4):1214–20.
Hoshino K, Hayakawa M, Morimoto Y. Minocycline prevents the impairment of hippocampal long-term potentiation in the septic mouse. Shock. 2017;48(2):209–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Chiara Robba, Ilaria Alice Crippa, and Fabio Silvio Taccone each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Critical Care
Rights and permissions
About this article
Cite this article
Robba, C., Crippa, I.A. & Taccone, F.S. Septic Encephalopathy. Curr Neurol Neurosci Rep 18, 82 (2018). https://doi.org/10.1007/s11910-018-0895-6
Published:
DOI: https://doi.org/10.1007/s11910-018-0895-6