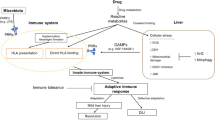
Abstract
Purpose of Review
With its high variability in both presentation and severity, drug-induced liver injury (DILI) is a complex condition increasingly confronting all providers. DILI has an even more muddled presentation among the geriatric population due to age-related changes in liver physiology and biochemistry as well as polypharmacy common in the geriatric population.
Recent Findings
Most cases of DILI are idiosyncratic and unpredictable. DILI, especially related to herbal and dietary supplement (HDS) use, is increasingly recognized as a leading cause of acute liver failure and need for liver transplantation. Unfortunately, liver transplantation is a limited option for the elderly, a population that exhibits significant HDS use. One recent study suggests that early use of N-acetylcysteine may be useful in preventing progression to acute liver failure in non-acetaminophen DILI. In the future, a personalized medicine approach using genomic signatures may be feasible to prevent DILI.
Summary
This review serves to raise recognition of the unique aspects of DILI in the geriatric population to promote rapid diagnosis and early intervention to prevent progression to liver failure and death. For now, DILI remains a diagnosis of exclusion, and care providers for the elderly must focus on obtaining a thorough history that includes HDS use and intervening early in suspected DILI cases.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Bower WA, Johns M, Margolis HS, Williams IT, Bell BP. Population-based surveillance for acute liver failure. Am J Gastroenterol. 2007;102(11):2459–63. https://doi.org/10.1111/j.1572-0241.2007.01388.x.
Chayanupatkul M, Schiano TD. Acute liver failure secondary to drug-induced liver injury. Clin Liver Dis. 2020;24(1):75–87. https://doi.org/10.1016/j.cld.2019.09.005.
United Nations DoEaSA, Population Division (2019). World Population Prospects 2019: Highlights.
Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC. Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med. 2016;176(4):473–82. https://doi.org/10.1001/jamainternmed.2015.8581.
Moore TJ, Cohen MR, Furberg CD. Serious adverse drug events reported to the Food and Drug Administration, 1998–2005. Arch Intern Med. 2007;167(16):1752–9. https://doi.org/10.1001/archinte.167.16.1752.
Schmucker DL. Age-related changes in liver structure and function: implications for disease ? Exp Gerontol. 2005;40(8–9):650–9. https://doi.org/10.1016/j.exger.2005.06.009.
Cieslak KP, Baur O, Verheij J, Bennink RJ, van Gulik TM. Liver function declines with increased age. HPB (Oxford). 2016;18(8):691–6. https://doi.org/10.1016/j.hpb.2016.05.011.
Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol. 2015;31(3):184–91. https://doi.org/10.1097/MOG.0000000000000176.
Andrade RJ, Chalasani N, Bjornsson ES, Suzuki A, Kullak-Ublick GA, Watkins PB, et al. Drug-induced liver injury. Nat Rev Dis Primers. 2019;5(1):58. https://doi.org/10.1038/s41572-019-0105-0.
Chen M, Suzuki A, Borlak J, Andrade RJ, Lucena MI. Drug-induced liver injury: interactions between drug properties and host factors. J Hepatol. 2015;63(2):503–14. https://doi.org/10.1016/j.jhep.2015.04.016.
Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging. 2018;4(4):267–85. https://doi.org/10.3233/NHA-170030.
Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46(11):1323–30. https://doi.org/10.1016/0895-4356(93)90101-6.
Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs--II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol. 1993;46(11):1331–6. https://doi.org/10.1016/0895-4356(93)90102-7.
Lucena MI, Andrade RJ, Kaplowitz N, Garcia-Cortes M, Fernandez MC, Romero-Gomez M, et al. Phenotypic characterization of idiosyncratic drug-induced liver injury: the influence of age and sex. Hepatology. 2009;49(6):2001–9. https://doi.org/10.1002/hep.22895.
Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148(7):1340–52.e7. https://doi.org/10.1053/j.gastro.2015.03.006.
Bjornsson ES, Bergmann OM, Bjornsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144(7):1419–25, 25.e1–3; quiz e19–20. https://doi.org/10.1053/j.gastro.2013.02.006.
Lucena MI, Andrade RJ, Fernandez MC, Pachkoria K, Pelaez G, Duran JA, et al. Determinants of the clinical expression of amoxicillin-clavulanate hepatotoxicity: a prospective series from Spain. Hepatology. 2006;44(4):850–6. https://doi.org/10.1002/hep.21324.
Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128(1):116–23. https://doi.org/10.1378/chest.128.1.116.
Holmberg L, Boman G, Bottiger LE, Eriksson B, Spross R, Wessling A. Adverse reactions to nitrofurantoin. Analysis of 921 reports. Am J Med. 1980;69(5):733–8. https://doi.org/10.1016/0002-9343(80)90443-x.
Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet. 2007;370(9582):173–84. https://doi.org/10.1016/s0140-6736(07)61091-5.
Page RL 2nd, Ruscin JM. The risk of adverse drug events and hospital-related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacother. 2006;4(4):297–305. https://doi.org/10.1016/j.amjopharm.2006.12.008.
Hanlon JT, Pieper CF, Hajjar ER, Sloane RJ, Lindblad CI, Ruby CM, et al. Incidence and predictors of all and preventable adverse drug reactions in frail elderly persons after hospital stay. J Gerontol A Biol Sci Med Sci. 2006;61(5):511–5. https://doi.org/10.1093/gerona/61.5.511.
Onder G, Pedone C, Landi F, Cesari M, Della Vedova C, Bernabei R, et al. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J Am Geriatr Soc. 2002;50(12):1962–8. https://doi.org/10.1046/j.1532-5415.2002.50607.x.
Nobili A, Pasina L, Tettamanti M, Lucca U, Riva E, Marzona I, et al. Potentially severe drug interactions in elderly outpatients: results of an observational study of an administrative prescription database. J Clin Pharm Ther. 2009;34(4):377–86. https://doi.org/10.1111/j.1365-2710.2009.01021.x.
• Guzman JR, Paterniti DA, Liu Y, Tarn DM. 2019, Factors related to disclosure and nondisclosure of dietary supplements in primary care, integrative medicine, and naturopathic medicine. J Fam Med Dis Prev. 5(4). doi:https://doi.org/10.23937/2469-5793/1510109. Provider inquiry is crucial in the disclosure of supplement use by patients. Nondisclosure factors include lack of provider inquiry, supplements being unrelated to the visit purpose, patients’ convictions that supplements are safe.
Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950–66; quiz 67. https://doi.org/10.1038/ajg.2014.131.
• Ahmad J, Reddy KR, Tillmann HL, Hayashi PH, Chalasani N, Fontana RJ, et al. Importance of hepatitis C virus RNA testing in patients with suspected drug-induced liver injury. Dig Dis Sci. 2019;64(9):2645–52. https://doi.org/10.1007/s10620-019-05591-w. 1.5% of DILI cases in the US DILI Network were actually acute hepatitis C infection cases. It is important to check HCV RNA in the diagnostic approach to DILI.
Grewal P, Ahmad J. Beware of HCV and HEV in patients with suspected drug-induced liver injury. Curr Hepatol Rep. 2018;17(3):270–5. https://doi.org/10.1007/s11901-018-0410-1.
Manka P, Bechmann LP, Coombes JD, Thodou V, Schlattjan M, Kahraman A, et al. Hepatitis E virus infection as a possible cause of acute liver failure in Europe. Clin Gastroenterol Hepatol. 2015;13(10):1836–42.e2; quiz e157–8. https://doi.org/10.1016/j.cgh.2015.04.014.
Spengler EK, Fontana RJ. Hepatitis E: emerging pathogen or domestic bystander? Clin Gastroenterol Hepatol. 2015;13(10):1843–5. https://doi.org/10.1016/j.cgh.2015.06.006.
Fontana RJ, Engle RE, Scaglione S, Araya V, Shaikh O, Tillman H, et al. The role of hepatitis E virus infection in adult Americans with acute liver failure. Hepatology. 2016;64(6):1870–80. https://doi.org/10.1002/hep.28649.
Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89(6):806–15. https://doi.org/10.1038/clpt.2011.58.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
Fontana RJ, Hayashi PH, Barnhart H, Kleiner DE, Reddy KR, Chalasani N, et al. Persistent liver biochemistry abnormalities are more common in older patients and those with cholestatic drug induced liver injury. Am J Gastroenterol. 2015;110(10):1450–9. https://doi.org/10.1038/ajg.2015.283.
Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, et al. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology. 2009;137(3):856–64, 64.e1. https://doi.org/10.1053/j.gastro.2009.06.006.
Bjornsson E, Talwalkar J, Treeprasertsuk S, Kamath PS, Takahashi N, Sanderson S, et al. Drug-induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010;51(6):2040–8. https://doi.org/10.1002/hep.23588.
Andrade RJ, Robles M, Lucena MI. Rechallenge in drug-induced liver injury: the attractive hazard. Expert Opin Drug Saf. 2009;8(6):709–14. https://doi.org/10.1517/14740330903397378.
Regev A, Bjornsson ES. Drug-induced liver injury: morbidity, mortality, and Hy’s law. Gastroenterology. 2014;147(1):20–4. https://doi.org/10.1053/j.gastro.2014.05.027.
Hunt CM, Yuen NA, Stirnadel-Farrant HA, Suzuki A. Age-related differences in reporting of drug- associated liver injury: data-mining of WHO Safety Report Database. Regul Toxicol Pharmacol. 2014;70(2):519–26. https://doi.org/10.1016/j.yrtph.2014.09.007.
Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev. 2009;8(4):339–48. https://doi.org/10.1016/j.arr.2009.06.001.
Bjornsson ES. Epidemiology, predisposing factors, and outcomes of drug-induced liver injury. Clin Liver Dis. 2020;24(1):1–10. https://doi.org/10.1016/j.cld.2019.08.002.
Barkin RL. Acetaminophen, aspirin, or ibuprofen in combination analgesic products. Am J Ther. 2001;8(6):433–42. https://doi.org/10.1097/00045391-200111000-00008.
Rotella JA, Wong A, Howell J, Robotham A, Greene S. High-visibility warning labels on paracetamol-containing products do not prevent supratherapeutic ingestion in a simulated scenario. Clin Toxicol (Phila). 2015;53(10):935–40. https://doi.org/10.3109/15563650.2015.1098657.
Lucena MI, Molokhia M, Shen Y, Urban TJ, Aithal GP, Andrade RJ, et al. Susceptibility to amoxicillin- clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141(1):338–47. https://doi.org/10.1053/j.gastro.2011.04.001.
Nicoletti P, Aithal GP, Chamberlain TC, Coulthard S, Alshabeeb M, Grove JI, et al. Drug-induced liver injury due to flucloxacillin: relevance of multiple human leukocyte antigen alleles. Clin Pharmacol Ther. 2019;106(1):245–53. https://doi.org/10.1002/cpt.1375.
Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Bjornsson ES, Chalasani N, et al. A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;156(6):1707–16.e2. https://doi.org/10.1053/j.gastro.2019.01.034.
Suh JI, Sakong JK, Lee K, Lee YK, Park JB, Kim DJ, et al. Anxiety and depression propensities in patients with acute toxic liver injury. World J Gastroenterol. 2013;19(47):9069–76. https://doi.org/10.3748/wjg.v19.i47.9069.
Alkharabsheh O, Kannarkatt P, Kannarkatt J, Karapetyan L, Laird-Fick HS, Al-Janadi A. An overview of the toxicities of checkpoint inhibitors in older patients with cancer. J Geriatr Oncol. 2018;9(5):451–8. https://doi.org/10.1016/j.jgo.2018.02.002.
van Holstein Y, Kapiteijn E, Bastiaannet E, van den Bos F, Portielje J, de Glas NA. Efficacy and adverse events of immunotherapy with checkpoint inhibitors in older patients with cancer. Drugs Aging. 2019;36(10):927–38. https://doi.org/10.1007/s40266-019-00697-2.
Kanesvaran R, Cordoba R, Maggiore R. Immunotherapy in older adults with advanced cancers: implications for clinical decision-making and future research. Am Soc Clin Oncol Educ Book. 2018;38:400–14. https://doi.org/10.1200/edbk_201435.
Wong A, Williams M, Milne D, Morris K, Lau P, Spruyt O, et al. Clinical and palliative care outcomes for patients of poor performance status treated with antiprogrammed death-1 monoclonal antibodies for advanced melanoma. Asia Pac J Clin Oncol. 2017;13(6):385–90. https://doi.org/10.1111/ajco.12702.
Grewal P, Ahmad J. Severe liver injury due to herbal and dietary supplements and the role of liver transplantation. World J Gastroenterol. 2019;25(46):6704–12. https://doi.org/10.3748/wjg.v25.i46.6704.
• Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, et al. Incidence and etiology of drug-induced liver injury in Mainland China. Gastroenterology. 2019;156(8):2230–41.e11. https://doi.org/10.1053/j.gastro.2019.02.002. In the largest registry evaluating DILI in Mainland China, there was a high incidence of HDS use (27%). Older patients were more likely to have elevations in liver tests and fatal DILI compared to younger patients. This study also showed similar patterns of chronic DILI and a cholestatic pattern of DILI in older patients.
Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 2002;25(5):323–44. https://doi.org/10.2165/00002018-200225050-00003.
Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399–408. https://doi.org/10.1002/hep.27317.
Medina-Caliz I, Garcia-Cortes M, Gonzalez-Jimenez A, Cabello MR, Robles-Diaz M, Sanabria-Cabrera J, et al. Herbal and dietary supplement-induced liver injuries in the Spanish DILI registry. Clin Gastroenterol Hepatol. 2018;16(9):1495–502. https://doi.org/10.1016/j.cgh.2017.12.051.
Suk KT, Kim DJ, Kim CH, Park SH, Yoon JH, Kim YS, et al. A prospective nationwide study of drug-induced liver injury in Korea. Am J Gastroenterol. 2012;107(9):1380–7. https://doi.org/10.1038/ajg.2012.138.
de Souza Silva JE, Santos Souza CA, da Silva TB, Gomes IA, Brito Gde C, de Souza Araujo AA, et al. Use of herbal medicines by elderly patients: a systematic review. Arch Gerontol Geriatr. 2014;59(2):227–33. https://doi.org/10.1016/j.archger.2014.06.002.
Raji MA, Kuo YF, Snih SA, Sharaf BM, Loera JA. Ethnic differences in herb and vitamin/mineral use in the elderly. Ann Pharmacother. 2005;39(6):1019–23. https://doi.org/10.1345/aph.1E506.
Yamashiki N, Sugawara Y, Tamura S, Nakayama N, Oketani M, Umeshita K, et al. Outcomes after living donor liver transplantation for acute liver failure in Japan: results of a nationwide survey. Liver Transpl. 2012;18(9):1069–77. https://doi.org/10.1002/lt.23469.
Gil E, Kim JM, Jeon K, Park H, Kang D, Cho J, et al. Recipient age and mortality after liver transplantation: a population-based cohort study. Transplantation. 2018;102(12):2025–32. https://doi.org/10.1097/tp.0000000000002246.
Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89(1):95–106. https://doi.org/10.1016/j.mayocp.2013.09.016.
Author information
Authors and Affiliations
Contributions
Lee: initial draft of the manuscript, approved the final draft to be submitted. Odin: revised draft of the manuscript, approved the final draft to be submitted. Grewal: revised draft of the manuscript, approved the final draft to be submitted.
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Conflict of Interest
The authors declare no competing interests.
Additional information
This article is part of the Topical Collection on Gastroenterology in Geriatrics Patients
Rights and permissions
About this article
Cite this article
Lee, B.T., Odin, J.A. & Grewal, P. An Approach to Drug-Induced Liver Injury from the Geriatric Perspective. Curr Gastroenterol Rep 23, 6 (2021). https://doi.org/10.1007/s11894-021-00804-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11894-021-00804-7