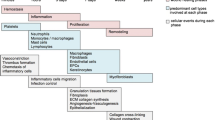
Abstract
Purpose of Review
Diabetic foot ulcerations (DFU) affect 25% of patients with diabetes mellitus during their lifetime and constitute a major health problem as they are often recalcitrant to healing due to a constellation of both intrinsic and extrinsic factors. The purpose of this review is to (1) detail the current mechanistic understanding of DFU formation and (2) highlight future therapeutic targets.
Recent Findings
From a molecular perspective, DFUs exhibit a chronic inflammatory predisposition. In addition, increased local hypoxic conditions and impaired cellular responses to hypoxia are pathogenic factors that contribute to delayed wound healing. Finally, recent evidence suggests a role for epigenetic alterations, including microRNAs, in delayed DFU healing due to the complex interplay between genes and the environment.
Summary
In this regard, notable progress has been made in the molecular and genetic understanding of DFU formation. However, further studies are needed to translate preclinical investigations into clinical therapies.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Centers for Diesease Control and Prevention 2014 National Diabetes Statistics Report; 2014.
Guariguata L, Whiting D, Weil C, Unwin N. The International Diabetes Federation diabetes atlas methodology for estimating global and national prevalence of diabetes in adults. Diabetes Res Clin Pract. 2011;94(3):322–32. https://doi.org/10.1016/j.diabres.2011.10.040.
Ray JA, Valentine WJ, Secnik K, Oglesby AK, Cordony A, Gordois A, et al. Review of the cost of diabetes complications in Australia, Canada, France, Germany, Italy and Spain. Curr Med Res Opin. 2005;21(10):1617–29. https://doi.org/10.1185/030079905X65349.
Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367–75. https://doi.org/10.1056/NEJMra1615439.
Lamont P, Franklyn K, Rayman G, Boulton AJM. Update on the diabetic foot 2012: the 14th biennial malvern diabetic foot conference, May 9-11, 2012. Int J Low Extrem Wounds. 2013;12(1):71–5. https://doi.org/10.1177/1534734613476519.
Braun LR, Fisk WA, Lev-Tov H, Kirsner RS, Isseroff RR. Diabetic foot ulcer: an evidence-based treatment update. Am J Clin Dermatol. 2014;15(3):267–81. https://doi.org/10.1007/s40257-014-0081-9.
Galkowska H, Wojewodzka U, Olszewski WL. Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen. 2006;14(5):558–65. https://doi.org/10.1111/j.1743-6109.2006.00155.x.
Goren I, Müller E, Pfeilschifter J, Frank S. Severely impaired insulin signaling in chronic wounds of diabetic ob/ob mice: a potential role of tumor necrosis factor-alpha. Am J Pathol. 2006;168(3):765–77. https://doi.org/10.2353/ajpath.2006.050293.
Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45(7):1011–6. https://doi.org/10.1007/s00125-002-0868-8.
Bakker K, Schaper NC, International Working Group on Diabetic Foot Editorial Board. The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28:116–8. https://doi.org/10.1002/dmrr.2254.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70. https://doi.org/10.1161/CIRCRESAHA.110.223545.
Pop-Busui R, Boulton AJM, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–54. https://doi.org/10.2337/dc16-2042.
Lavery LA, Peters EJG, Williams JR, Murdoch DP, Hudson A, Lavery DC, et al. Reevaluating the way we classify the diabetic foot: restructuring the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care. 2008;31(1):154–6. https://doi.org/10.2337/dc07-1302.
Dinh TL, Veves A. A review of the mechanisms implicated in the pathogenesis of the diabetic foot. Int J Low Extrem Wounds. 2005;4(3):154–9. https://doi.org/10.1177/1534734605280130.
Dinh T, Veves A. Microcirculation of the diabetic foot. Curr Pharm Des. 2005;11(18):2301–9. https://doi.org/10.2174/1381612054367328.
LoGerfo FW, Coffman JD. Vascular and microvascular disease of the foot in diabetes. N Engl J Med. 1984;311(25):1615–9. https://doi.org/10.1056/NEJM198412203112506.
Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21. https://doi.org/10.1038/nature07039.
Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122(18):3209–13. https://doi.org/10.1242/jcs.031187.
Wicks K, Torbica T, Mace KA. Myeloid cell dysfunction and the pathogenesis of the diabetic chronic wound. Semin Immunol. 2014;26(4):341–53. https://doi.org/10.1016/j.smim.2014.04.006.
• Mirza RE, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes. 2013;62(7):2579–87. This study demonstrated IL-1β is significantly increased in diabetic wounds as well as targeted IL-1β inhibition (using both pharmacologic or genetic models) improves the rate of diabetic wound healing. https://doi.org/10.2337/db12-1450.
Dinh T, Tecilazich F, Kafanas A, Doupis J, Gnardellis C, Leal E, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61(11):2937–47. https://doi.org/10.2337/db12-0227.
Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63(3):1103–14. https://doi.org/10.2337/db13-0927.
Mirza RE, Fang MM, Novak ML, Urao N, Sui A, Ennis WJ, et al. Macrophage PPARγ and impaired wound healing in type 2 diabetes. J Pathol. 2015;236(4):433–44. https://doi.org/10.1002/path.4548.
Falanga V. Wound healing and its impairment in the diabetic foot. Lancet (London, England). 2005;366(9498):1736–43. https://doi.org/10.1016/S0140-6736(05)67700-8.
Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther. 2014;31(8):817–36. https://doi.org/10.1007/s12325-014-0140-x.
Bannon P, Wood S, Restivo T, Campbell L, Hardman MJ, Mace KA. Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing in mice. Dis Model Mech. 2013;6(6):1434–47. https://doi.org/10.1242/dmm.012237.
Finley PJ, DeClue CE, Sell SA, DeBartolo JM, Shornick LP. Diabetic wounds exhibit decreased Ym1 and arginase expression with increased expression of IL-17 and IL-20. Adv wound care. 2016;5(11):486–94. https://doi.org/10.1089/wound.2015.0676.
•• Guo Y, Lin C, Xu P, Wu S, Fu X, Xia W, et al. AGEs induced autophagy impairs cutaneous wound healing via stimulating macrophage polarization to M1 in diabetes. Sci Rep. 2016;6(1):36416. This study demonstrates in human tissue and murine model that enhanced autophagy negatively impacts wound healing and that pharmacological inhibition of autophagy improves diabetic wound healing. https://doi.org/10.1038/srep36416.
• Wang Y, Xiao Y, Zhong L, Ye D, Zhang J, Tu Y, et al. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with-cell autoimmunity in patients with type 1 diabetes. Diabetes. 2014;63(12):4239–48. This study supports an early role of neutrophil activation and augmented neutrophil serine proteases activities in the pathogenesis of type I diabetes and diabetic wound progression. https://doi.org/10.2337/db14-0480.
Menegazzo L, Ciciliot S, Poncina N, Mazzucato M, Persano M, Bonora B, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 2015;52(3):497–503. https://doi.org/10.1007/s00592-014-0676-x.
•• Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med. 2015;21(7):815–9. This study is one of the first to demonstrae in human tissue and murine model that neutrophil extracellular traps (NETs) are increased in diabetic wounds in comparison to control tissue. In addition, genetic and pharamcological inhibition of NETosis improved wound healing. https://doi.org/10.1038/nm.3887.
Semenza G. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. https://doi.org/10.1016/j.cell.2012.01.021.
Zhang X, Liu L, Wei X, Tan YS, Tong L, Chang, BS R, et al. Impaired angiogenesis and mobilization of circulating angiogenic cells in HIF-1alpha heterozygous-null mice after burn wounding. Wound Repair Regen. 2010;18(2):193–201. https://doi.org/10.1111/j.1524-475X.2010.00570.x.
Botusan IR, Sunkari VG, Savu O, Catrina AI, Grunler J, Lindberg S, et al. Stabilization of HIF-1alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105(49):19426–31. https://doi.org/10.1073/pnas.0805230105.
Mace KA, Yu DH, Paydar KZ, Boudreau N, Young DM. Sustained expression of Hif-1alpha in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen. 2007;15(5):636–45. https://doi.org/10.1111/j.1524-475X.2007.00278.x.
Catrina S-B, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes. 2004;53(12):3226–32. https://doi.org/10.2337/diabetes.53.12.3226.
Bento CF, Fernandes R, Ramalho J, Marques C, Shang F, Taylor A, et al. The chaperone-dependent ubiquitin ligase CHIP targets HIF-1α for degradation in the presence of methylglyoxal. PLoS One. 2010;5(11):e15062. https://doi.org/10.1371/journal.pone.0015062.
Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, et al. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008;283(16):10930–8. https://doi.org/10.1074/jbc.M707451200.
Sunkari VG, Lind F, Botusan IR, Kashif A, Liu Z-J, Ylä-Herttuala S, et al. Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen. 2015;23(1):98–103. https://doi.org/10.1111/wrr.12253.
Liu L, Marti GP, Wei X, Zhang X, Zhang H, Liu YV, et al. Age-dependent impairment of HIF-1alpha expression in diabetic mice: correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008;217(2):319–27. https://doi.org/10.1002/jcp.21503.
Gu HF, Zheng X, Abu Seman N, Gu T, Botusan IR, Sunkari VG, et al. Impact of the hypoxia-inducible factor-1 α (HIF1A) Pro582Ser polymorphism on diabetes nephropathy. Diabetes Care. 2013;36(2):415–21. https://doi.org/10.2337/dc12-1125.
Rundhaug JE. Matrix metalloproteinases and angiogenesis. J Cell Mol Med. 2005;9(2):267–85. https://doi.org/10.1111/j.1582-4934.2005.tb00355.x.
Cawston TE, Wilson AJ. Understanding the role of tissue degrading enzymes and their inhibitors in development and disease. Best Pract Res Clin Rheumatol. 2006;20(5):983–1002. https://doi.org/10.1016/j.berh.2006.06.007.
Hopps E, Lo Presti R, Montana M, Noto D, Averna MR, Caimi G. Gelatinases and their tissue inhibitors in a group of subjects with metabolic syndrome. J Investig Med. 2013;61(6):978–83. https://doi.org/10.2310/JIM.0b013e318294e9da.
Gibson DJ, Schultz GS. Molecular wound assessments: matrix metalloproteinases. Adv Wound Care. 2013;2(1):18–23. https://doi.org/10.1089/wound.2011.0359.
Singh K, Agrawal NK, Gupta SK, Mohan G, Chaturvedi S, Singh K. Differential expression of matrix metalloproteinase-9 gene in wounds of type 2 diabetes mellitus cases with susceptible -1562C>T genotypes and wound severity. Int J Low Extrem Wounds. 2014;13(2):94–102. https://doi.org/10.1177/1534734614534980.
Widgerow AD. Chronic wound fluid--thinking outside the box. Wound Repair Regen. 2011;19(3):287–91. https://doi.org/10.1111/j.1524-475X.2011.00683.x.
Gooyit M, Peng Z, Wolter WR, Pi H, Ding D, Hesek D, et al. A chemical biological strategy to facilitate diabetic wound healing. ACS Chem Biol. 2014;9(1):105–10. https://doi.org/10.1021/cb4005468.
Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996;4(4):411–20. https://doi.org/10.1046/j.1524-475X.1996.40404.x.
Berezin A. Metabolic memory phenomenon in diabetes mellitus: achieving and perspectives. Diabetes Metab Syndr. 2016;10(2):S176–83. https://doi.org/10.1016/j.dsx.2016.03.016.
Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58(3):443–55. https://doi.org/10.1007/s00125-014-3462-y.
Park LK, Maione AG, Smith A, Gerami-Naini B, Iyer LK, Mooney DJ, et al. Genome-wide DNA methylation analysis identifies a metabolic memory profile in patient-derived diabetic foot ulcer fibroblasts. Epigenetics. 2014;9(10):1339–49. https://doi.org/10.4161/15592294.2014.967584.
•• Gallagher KA, Joshi A, Carson WF, Schaller M, Allen R, Mukerjee S, et al. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes. 2015;64(4):1420–30. This study demonstrated that in diabetic patient samples and murine model, the epigenetic enzyme, JMJD3, demethylates repressive histone markers on the IL-12 promoter resulting in increased inflammatory gene expression in bone marrow progenitor cells, circulating blood monocytes, and tissue macrophages. https://doi.org/10.2337/db14-0872.
Liu Y-F, Ding M, Liu D-W, Liu Y, Mao Y-G, Peng Y. MicroRNA profiling in cutaneous wounds of diabetic rats. Genet Mol Res. 2015;14(3):9614–25. https://doi.org/10.4238/2015.August.14.24.
Madhyastha R, Madhyastha H, Nakajima Y, Omura S, Maruyama M. MicroRNA signature in diabetic wound healing: promotive role of miR-21 in fibroblast migration. Int Wound J. 2012;9(4):355–61. https://doi.org/10.1111/j.1742-481X.2011.00890.x.
Li Y, Reddy MA, Miao F, Shanmugam N, Yee J-K, Hawkins D, et al. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem. 2008;283(39):26771–81. https://doi.org/10.1074/jbc.M802800200.
Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3(3):251–5. https://doi.org/10.1007/s12265-010-9169-7.
Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the microRNA perspective. Diabetes. 2011;60(7):1832–7. https://doi.org/10.2337/db11-0082.
Palmero EI, de Campos SGP, Campos M, de Souza NCN, Guerreiro IDC, Carvalho AL, et al. Mechanisms and role of microRNA deregulation in cancer onset and progression. Genet Mol Biol. 2011;34(3):363–70. https://doi.org/10.1590/S1415-47572011000300001.
Liu J, Xu Y, Shu B, Wang P, Tang J, Chen L, et al. Quantification of the differential expression levels of microRNA-203 in different degrees of diabetic foot. Int J Clin Exp Pathol. 2015;8(10):13416–20.
• Game FL, Apelqvist J, Attinger C, Hartemann A, Hinchliffe RJ, Löndahl M, et al. Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(Suppl 1):154–68. This review provides a detailed summary of current consencious guidelines for the treatment of diabetic foot ulcers. https://doi.org/10.1002/dmrr.2707.
Garwood CS, Steinberg JS. What’s new in wound treatment: a critical appraisal. Diabetes Metab Res Rev. 2016;32:268–74. https://doi.org/10.1002/dmrr.2747.
Wu Y, Wang J, Scott PG, Tredget EE. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007;15(Suppl 1):S18–26. https://doi.org/10.1111/j.1524-475X.2007.00221.x.
• Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, et al. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011;92(1):26–36. This study is a randomized clinical study on 41 limnbs investigating the differential administration of bone marrow mesenchymal stem cells and bone marrow-derived mononuclear cells on the morbidity and healing rates of diabetic foot ulcers. https://doi.org/10.1016/j.diabres.2010.12.010.
Xu S-M, Liang T. Clinical observation of the application of autologous peripheral blood stem cell transplantation for the treatment of diabetic foot gangrene. Exp Ther Med. 2016;11(1):283–8. https://doi.org/10.3892/etm.2015.2888.
Moura J, Børsheim E, Carvalho E. The role of microRNAs in diabetic complications-special emphasis on wound healing. Genes (Basel). 2014;5(4):926–56. https://doi.org/10.3390/genes5040926.
Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15(2):261–71. https://doi.org/10.1016/j.devcel.2008.07.002.
Ortega FJ, Mercader JM, Moreno-Navarrete JM, Rovira O, Guerra E, Esteve E, et al. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care. 2014;37(5):1375–83. https://doi.org/10.2337/dc13-1847.
van Solingen C, Seghers L, Bijkerk R, Duijs JMGJ, Roeten MK, van Oeveren-Rietdijk AM, et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med. 2009;13(8a):1577–85. https://doi.org/10.1111/j.1582-4934.2008.00613.x.
Nesca V, Guay C, Jacovetti C, Menoud V, Peyot M-L, Laybutt DR, et al. Identification of particular groups of microRNAs that positively or negatively impact on beta cell function in obese models of type 2 diabetes. Diabetologia. 2013;56(10):2203–12. https://doi.org/10.1007/s00125-013-2993-y.
Nielsen LB, Wang C, Sørensen K, et al. Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp Diabetes Res. 2012;2012:896362.
Greco S, Fasanaro P, Castelvecchio S, D’Alessandra Y, Arcelli D, Di Donato M, et al. MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes. 2012;61(6):1633–41. https://doi.org/10.2337/db11-0952.
Pastar I, Khan AA, Stojadinovic O, Lebrun EA, Medina MC, Brem H, et al. Induction of specific microRNAs inhibits cutaneous wound healing. J Biol Chem. 2012;287(35):29324–35. https://doi.org/10.1074/jbc.M112.382135.
Shu Y, Pi F, Sharma A, Rajabi M, Haque F, Shu D, et al. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Adv Drug Deliv Rev. 2014;66:74–89. https://doi.org/10.1016/j.addr.2013.11.006.
• Icli B, Nabzdyk CS, Lujan-Hernandez J, Cahill M, Auster ME, Wara AKM, et al. Regulation of impaired angiogenesis in diabetic dermal wound healing by microRNA-26a. J Mol Cell Cardiol. 2016;91:151–9. This study demonstrated that microRNA-26a is significantly upregulated in murine diabetic wounds and adminstration of a miR-26a inhibitor significantly increased rate of wound healing. https://doi.org/10.1016/j.yjmcc.2016.01.007.
Wang J-M, Tao J, Chen D-D, Cai J-J, Irani K, Wang Q, et al. MicroRNA miR-27b rescues bone marrow-derived angiogenic cell function and accelerates wound healing in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34(1):99–109. https://doi.org/10.1161/ATVBAHA.113.302104.
Acknowledgements
We would like to thank Robin Kunkel for her assistance with the scientific illustrations provided in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Frank M. Davis, Andrew Kimball, Anna Boniakowski, and Katherine Gallagher declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Microvascular Complications—Neuropathy
Rights and permissions
About this article
Cite this article
Davis, F.M., Kimball, A., Boniakowski, A. et al. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr Diab Rep 18, 2 (2018). https://doi.org/10.1007/s11892-018-0970-z
Published:
DOI: https://doi.org/10.1007/s11892-018-0970-z