Abstract
Purpose of Review
This review summarizes the evidence regarding diet, physical activity, smoking, and body composition after colorectal cancer (CRC) diagnosis in relation to all-cause and CRC-specific mortality and disease recurrence and gives suggestions for future research directions.
Recent Findings
Overall, this review suggests that some, albeit not all, of the well-known modifiable risk factors for cancer incidence might also be associated with CRC survival. CRC prognosis appears to be worse with increased physical inactivity, smoking, or being underweight after CRC diagnosis. Emerging evidence suggests that diets associated with a positive energy balance, e.g., high consumption of sugar-sweetened beverages, may negatively impact survival in CRC survivors. In contrast, there is currently little evidence to support the recommendation to limit red and processed meat or alcohol intake after CRC diagnosis. Whether being overweight and obese after CRC diagnosis improves or worsens CRC prognosis remains controversial and may depend on the measure used to assess body fatness.
Summary
Further research on post-diagnosis lifestyle patterns is needed to understand the multifactorial influence on CRC prognosis. Disease recurrence and the development of comorbidities should be included as key outcomes in future studies and lifestyle should preferably be repeatedly measured.
Similar content being viewed by others
Introduction
Diet, physical activity, smoking, alcohol, and body weight are associated with risk (incidence) of colorectal cancer (CRC) [1, 2]. In contrast, far fewer studies have examined the influence of these lifestyle factors on survival after CRC diagnosis. Currently, cancer survivors are advised to follow the recommendations formulated for cancer prevention [3]. However, it is currently unclear if making lifestyle changes after diagnosis would impact disease progression and survival.
Emerging evidence shows that lifestyle, including diet, after CRC diagnosis might affect all-cause and CRC-specific mortality risk. Several recent reviews and meta-analyses on observational studies summarized the available evidence on specific aspects of lifestyle, such as diet [4••, 5, 6], physical activity [4••, 5, 7,8,9,10, 11••, 12], smoking [13••, 14], and body composition [5, 10, 15, 16, 17••, 18,19,20,21,22], in relation to CRC outcomes. However, none of these reviews included all the aforementioned lifestyle factors in one review. Furthermore, results might differ due to the timing of lifestyle assessment (e.g., pre-diagnosis vs. post-diagnosis) [8, 10, 15] and characteristics of the included study population [15].
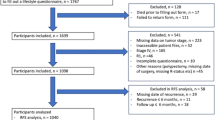
To better understand the association between lifestyle and CRC outcomes, we summarized the evidence regarding diet, physical activity, smoking, and body composition after CRC diagnosis across different groups of cancer survivors. Moreover, we also included observational studies, not included in previous reviews [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38, 39••]. We identified three study design categories based on the selection of the included study population: (1) population-based studies including all incident CRC cases, (2) studies in the adjuvant setting limited to survivors treated with adjuvant therapy, and (3) studies in the metastatic setting limited to patients with metastatic disease (Fig. 1). We chose to focus on post-diagnosis lifestyle factors, because this is the period during which CRC survivors could be counseled to alter their behavior. Therefore, we only included studies that examined the association between lifestyle at or after CRC diagnosis and all-cause mortality, CRC-specific mortality, or cancer recurrence. Additionally, we summarized the evidence regarding changes in lifestyle, i.e., from pre- to post-diagnosis or changes made after diagnosis, among CRC survivors and survival outcomes from either observational or intervention studies. We did not include papers that examined lifestyle and CRC survival separately by molecular subtypes. These publications will be reviewed in future issue of this journal. Finally, we conclude with suggestions for future research directions.
Schematic diagram of identification of three study categories based on the characteristics of the included study population. Based on the study population, studies were categorized into (1) population-based studies including all incident colorectal cancer cases, (2) studies in the adjuvant setting limited to survivors treated with adjuvant therapy, and (3) studies in the metastatic setting limited to metastatic patients. In each study category, we identified studies with lifestyle information available at or after colorectal cancer diagnosis. Studies with lifestyle information limited to the period before colorectal cancer diagnosis, either collected prospectively before diagnosis or retrospectively after diagnosis, were not taken into account
Overview of Included Studies
We excluded all studies that did not assess lifestyle at or after CRC diagnosis (e.g., those that assessed only pre-diagnosis factors) or did not adjust for critical confounders (e.g., age, stage). Furthermore, we excluded all studies that dichotomized body mass index (BMI) when examining the association between BMI and mortality or recurrence. Dichotomized BMI is considered a crude classification of BMI by combining diverse categories of body mass and body composition. Thus, dichotomized BMI may not account for potential differential associations between sub-categories of BMI (e.g., by combining overweight and obese in one category) [15].
We included 57 relevant articles (based on 84 different observational studies) that reported on post-diagnosis diet, physical activity, smoking, or body fatness/body composition in CRC survivors in relation to all-cause mortality, CRC-specific mortality, or cancer recurrence. An overview of the number of included articles according to exposure and type of study population is shown in Fig. 2. Additionally, we included 13 relevant articles (one intervention study and 11 different observational studies) that reported on changes in lifestyle among CRC survivors in relation to survival outcomes. In total, 61 articles are discussed in more detail in this review.
Overview of the number of included relevant articles on diet, physical activity, smoking and body mass index (BMI) or body composition at or after colorectal cancer diagnosis in relation to all-cause mortality, cancer-specific mortality, or disease recurrence by type of included study population. In total, 57 articles were included: 54 articles reported on one exposure, two articles reported on both physical activity and BMI, and one article reported on all four exposures
Diet after CRC Diagnosis
Five population-based studies and one study in the adjuvant setting provided results on diet and CRC outcomes in 10 publications [23,24,25,26,27, 40,41,42,43,44] (Table 1). Three US cohorts assessed post-diagnosis diet in population-based cohorts with > 1000 CRC patients: Nurses’ Health Study I (NHS) [23, 44], Health Professional Follow-Up Study (HPFS) [44], and Cancer Prevention Study (CPS) II Nutrition Cohort [27, 40, 41]. All three cohorts consist of participants diagnosed with CRC during follow-up and have updated dietary assessment after diagnosis. Usually, questionnaires that were completed after treatment was finished were utilized in the analyses. In contrast, two non-US cohorts (the German cohort PopGen [24] and BioBank Japan [26]) recruited > 1000 CRC patients after CRC diagnosis. The study in the adjuvant setting, Cancer and Leukemia Group B (CALGB) 89,803 Diet and Lifestyle Companion study [25, 42, 43], was embedded in a randomized trial of adjuvant chemotherapy among ~ 1000 patients with stage III colon cancer. Additionally, three articles, two from the CPS II Nutrition Cohort [27, 40] and one report on a small randomized dietary intervention trial reported on dietary changes among CRC survivors in relation to mortality [27, 40, 78].
In this review, we summarized the available evidence for dietary patterns, red and processed meat, sugar-sweetened beverages, alcohol consumption, other foods and beverages, and CRC survival.
Dietary Patterns
Two observational studies, the NHS I [23] and a German cohort of CRC survivors [24], assessed post-diagnosis dietary patterns in a population-based setting [23], while CALGB 89803 [42] reported results in the adjuvant setting (Table 1). Data-driven dietary patterns were assessed within NHS I [23] and CALGB 89803 [42]. Both studies observed patterns that were given the labels a “Western” and a “Prudent” dietary pattern. The Western dietary pattern was characterized by high- and low-fat dairy, refined grains, red and processed meats, desserts, and potatoes, while the Prudent dietary pattern was characterized by high intakes of fruits, vegetables, whole grains, and poultry.
For the Western dietary pattern, both studies reported an increased all-cause mortality risk [23, 42]. However, the association was statistically significant only in the adjuvant setting (CALBG: Q5 vs. Q1: HR 2.32; 95% CI 1.36–3.96; P trend <0.001) [42], and not in the population-based study (NHS I: Q5 vs. Q1: HR 1.32 (0.89–1.97); P trend = 0.23) [23]. Similarly, a statistically significant increased risk of colon cancer recurrence was reported in the adjuvant setting [42], while a non-significant positive association was reported for CRC mortality in the population-based study [23] (Table 1). For the Prudent dietary pattern, both studies reported statistically non-significant associations for all-cause mortality [23, 42], CRC-specific mortality [23], or colon cancer recurrence [42].
Furthermore, several a priori-defined dietary patterns were studied in the two population-based studies [23, 24] (Table 1). Of the a priori-defined dietary patterns, none has been studied in more than one cohort. Some a priori-defined dietary patterns were associated with lower risk of all-cause mortality, but not all [23, 24].
Only one small (n = 111) randomized dietary intervention trial among CRC survivors assessed associations with survival [78]. Throughout the 1.5 months of neoadjuvant radiotherapy patients with rectal cancer randomized to the intervention group received 6 weekly individualized nutrition counseling and education sessions using regular foods, while the control group maintained their usual diet. Overall, the main goal of the intervention was to enable every patient to achieve his or her calculated energy and protein requirements. After long-term follow-up (median follow-up 6.5 (range 4.9–8.1) years), CRC-specific survival was significantly longer in the intervention group after adjustment for age and disease stage (median survival 7.3 vs. 4.9 years).
Red and Processed Meats
Both NHS I [23] and CPS II Nutrition Cohort [40] reported on post-diagnosis red and processed meat intake, although the NHS I paper focused on dietary patterns (Table 1). The CPS II Nutrition Cohort also provided information regarding pre- to post-diagnosis change in red and processed meat consumption [40] (Table 2).
These two studies did not observe an association between red and processed meat intake and both all-cause mortality and CRC-specific mortality [23, 40]. Furthermore, changing meat intake from high (median or higher) before CRC diagnosis to low (below median) after diagnosis was not associated with lower mortality when compared to survivors with a consistently high intake [40].
Sugar-Sweetened Beverages
Both the NHS I [23] and CALGB 89803 [43] reported on post-diagnosis sugar-sweetened beverage intake and CRC outcomes (Table 1).
Both studies [23, 43] reported increased all-cause mortality risk for sugar-sweetened beverage consumption after CRC diagnosis, of which the association in the NHS I was statistically significant [23]. Each additional serving of sugar-sweetened beverages (including fruit juices) after CRC diagnosis was associated with an 11% increased risk for all-cause mortality (HR 1.11; 95% CI 1.01–1.23) [23]. A similar relative risk was reported for CRC-specific mortality, although it was not statistically significant [23]. For colon cancer recurrence, CALGB 89803 reported a statistically significant increased recurrence risk for patients consuming ≥ 2 servings of sugar-sweetened beverages per day (HR 1.75; 95% CI 1.04–2.94) compared to those consuming < 2 servings per month (P trend = 0.04) [43].
Alcohol
Four population-based studies, NHS I [23, 44], HPFS [44], CPS II Nutrition cohort [27], and a Japanese cohort of CRC survivors [26], reported on post-diagnosis alcohol consumption and CRC outcomes (Table 1).
In NHS I, moderate drinking was used as the reference group and abstaining from alcohol consumption was associated with a statistically significant increased all-cause mortality risk (HR 1.30; 1.05–1.61) compared to women consuming 5–15 g of alcohol per day [23]. Drinking > 15 g/day (approximately 1.5 drinks) was not statistically significantly associated with increased mortality risk. Similarly, abstainers had a higher mortality risk than drinkers in the Japanese cohort [26] and after combining both NHS I and HPFS cohort data [44]. However, the CPS II Nutrition cohort reported that drinking alcohol after diagnosis was not associated with all-cause mortality [27]. For CRC-specific mortality, similar results were reported as for all-cause mortality (Table 1).
The CPS II Nutrition cohort also provided information regarding pre- to post-diagnosis change in alcohol consumption (Table 2). Participants who reported drinking before CRC diagnosis but stopped drinking alcohol after diagnosis had a statistically non-significant increased risk of all-cause and CRC-specific mortality compared to participants who continued to drink alcohol [27].
Other Foods, Beverages, and Nutrients
The intake of some foods, beverages, and nutrients were only reported in one study each (Table 1). Higher nut consumption was associated with lower risk of CRC mortality (HR/serving/day 0.69; 95% CI 0.49–0.97) in the NHS I, while no statistically significant association was reported for all-cause mortality [23]. Furthermore, no associations were observed within the NHS I with either all-cause mortality or CRC-specific mortality for vegetables, fruits, or whole grains [23]. However, in the Japanese study, lower green leafy vegetable intake after CRC diagnosis was associated with an increased all-cause mortality risk [26].
Higher milk intake was statistically significantly associated with lower all-cause mortality risk (Q4 vs. Q1: HR 0.72; 95% CI 0.55–0.94; P trend = 0.02) in the CPS II Nutrition Cohort [41]. A similar risk was reported for overall dairy consumption, although associations did not reach statistical significance [41]. Additionally, higher coffee intake was statistically significantly associated with lower all-cause mortality (≥ 4 vs. 0 cups/day: HR 0.66; 95% CI 0.37–1.18; P trend = 0.01) within CALGB 89803 [25]. No significant associations were reported for non-herbal tea intake [25].
Higher dietary glycemic load and total carbohydrate intake were statistically significant associated with an increased risk of mortality and recurrence in CALGB 89803 [45]. Higher total calcium intake was statistically significantly associated with both lower all-cause mortality and CRC-specific mortality in the CPS II Nutrition Cohort, while no significant associations were reported for vitamin D [41]. Also no significant associations were reported for intake of one-carbon nutrients (folate, vitamins B6 and B12) in NHS I [44].
Diet: Key Points
One small randomized intervention trial which provided individualized nutritional counseling and education about regular foods suggest that making dietary changes may improve cancer-specific survival. No dietary pattern or food has been studied in more than two observational cohorts, with cancer recurrence only studied in one cohort in the adjuvant setting embedded in a randomized chemotherapy trial. While alcohol consumption has been studied more frequently, these studies often used abstainers as comparison group. Abstainers are probably an inappropriate reference group, as this group may, at least in part, include people who stopped drinking because of comorbidities or cancer-related symptoms. Overall, emerging evidence shows that diet after CRC diagnosis might affect survival, but further research is needed to clarify what aspects of diet are important and which dietary changes could affect survival.
Physical Activity after CRC Diagnosis
Seven population-based studies [26, 46,47,48,49,50,51] and one study in the adjuvant setting [52] provided results on physical activity after CRC diagnosis and mortality outcomes (Table 1). Five large US cohorts assessed post-diagnosis physical activity in population-based cohorts with > 500 CRC patients: NHS I [46], HPFS [47], CPS II Nutrition Cohort [50], Women’s Health Initiative [49], and National Institutes of Health-AARP Diet and Health Study [51]. All five cohorts consist of participants diagnosed with CRC during follow-up and have updated physical activity assessment after diagnosis, usually when treatment was completed. In contrast, two non-US cohorts (an Australian cohort [48] and BioBank Japan [26]) recruited > 1500 CRC patients after CRC diagnosis. All studies reported on leisure time physical activity.
Physical Activity
For all-cause mortality, seven studies [26, 46,47,48,49,50,51,52] were included in previous meta-analyses [7,8,9,10]. These meta-analyses have found highest versus lowest post-diagnostic physical activity to be associated with 40% lower all-cause mortality risk [7,8,9,10]. Five studies that were included in a dose-response meta-analysis showed a 28% lower risk of all-cause mortality (HR 0.72; 95% CI 0.65–0.80) for every 10 metabolic equivalent task-hour per week (MET-hours/week) increase in post-diagnosis physical activity [9], which is equivalent to current recommendations of 150 min/week of at least moderate intensity activity. For CRC-specific mortality, similar risk reductions were reported comparing high versus low physical activity after CRC diagnosis (HR 0.62; 95% CI 0.45–0.86) [11••] and for every 10 MET-hours/week increase in post-diagnosis physical activity (HR 0.75; 95% CI 0.65–0.85) [9].
Changes in Physical Activity
The Australian cohort [48] and NHS I [46] also provided results on changes in physical activity and mortality outcomes in CRC patients (Table 2). An increase of physical activity > 2 h/week between 5 and 12 months post-diagnosis was statistically significantly associated with lower all-cause (HR 0.69; 95% CI 0.50–0.94) and CRC-specific mortality (HR 0.64; 95% CI 0.44–0.93) among Australian CRC survivors [48]. A pre- to post-diagnosis increase in physical activity showed a statistically significant lower all-cause and CRC-specific mortality risk in the NHS I [46], but no association was reported among Australian CRC survivors [48] (Table 2). The first randomized controlled trial designed primarily to assess the impact of physical activity on survival among colon cancer survivors is ongoing [83]. As of April 2017, the trial has enrolled 536 of its planned 972 participants [84] and only 1 year feasibility results have been published so far [85].
Sedentary Behavior
Three of the population-based studies, CPS II Nutrition Cohort [50], National Institutes of Health-AARP Diet and Health [51], and HPFS [53] also reported on post-diagnosis sedentary behavior and all-cause as well as CRC-specific mortality (Table 1). CPS II reported on leisure time spent sitting [50], whereas the other two studies assessed TV viewing [51, 53]. All three studies [50, 51, 53] reported no statistically significant associations between sedentary behavior and all-cause mortality. With regard to CRC-specific mortality, only one study, the CPS II Nutrition Cohort showed a statistically significant positive association between sedentary behavior and CRC-specific mortality (≥ 6 h vs. < 3 h/day sitting time: HR 1.62; 95% CI 1.07–2.44) [50].
Physical Activity: Key Points
Evidence from prospective observational studies has consistently suggested that higher physical activity after CRC diagnosis is associated with a lower risk of CRC-specific and all-cause mortality, but whether physical activity is causally related to CRC mortality remains unclear. A randomized controlled trial is currently ongoing to address whether aerobic physical activity after complement of adjuvant therapy improves survival. Based on a few studies, there is some evidence suggesting that excessive sedentary behavior after CRC diagnosis might be associated with increased CRC-specific mortality, but findings are less consistent than for leisure time physical activity.
Smoking after CRC Diagnosis
Eleven population-based studies [14, 26, 28,29,30,31, 54,55,56,57,58] and three studies in the adjuvant setting [59,60,61] reported on smoking at or after CRC diagnosis and mortality outcomes (Table 1). Four population-based studies used data from a cancer registry [14, 30, 31, 55]; three were from single-institution hospital cohorts [54, 56, 57]; three were non-US cohorts (Shanghai Cohort Study [28], the German cohort DACHS [58], and BioBank Japan [26]); and lastly, the CPS II Nutrition cohort [29]. Two studies in the adjuvant setting were embedded in an adjuvant chemotherapy trial, CALGB 89803 [60] and N0147 [61], while the third study included patients referred to a single institution for consideration of adjuvant treatment [59]. Six studies [28, 31, 54, 57,58,59] compared current smokers with non-smokers, while eight studies [14, 26, 29, 30, 55, 56, 60, 61] compared current smokers with never smokers.
Smoking
For all-cause mortality, eight out of nine population-based studies [26, 28, 29, 31, 54,55,56,57] reported increased all-cause mortality risk for smoking, of which six [26, 28, 29, 54, 55, 57] were statistically significant. Furthermore, the study in the adjuvant setting also reported a statistically significant increased all-cause mortality risk for smoking [61].
For CRC-specific mortality, five population-based studies [14, 29, 30, 57, 58] reported increased CRC-specific mortality risk for smoking, of which three [14, 29, 30] were statistically significant (Table 1). However, one study that reported results separately for men and women reported a statistically non-significant positive association among women for post-diagnosis smoking, while among men, a statistically non-significant inverse association was reported [56]. Furthermore, one study in the adjuvant setting also reported a statistically significant increased CRC-specific mortality risk for smoking [59].
For colon cancer recurrence, one study embedded in the trial N0147 [61] reported a statistically significant increased cancer recurrence risk for smoking, while CALGB 89803 [60] reported no association with smoking among stage III colon cancer patients treated with adjuvant chemotherapy.
Smoking Cessation
Four population-based studies provided results on smoking cessation and mortality outcomes in CRC patients (Table 2). People who continued smoking after CRC diagnosis had a more than threefold increased risk of all-cause mortality (HR 3.46; 95% CI 1.69–7.10) compared to people who quit smoking after diagnosis [28]. Pre- to post-diagnosis smoking cessation was not statistically significantly associated with all-cause or CRC-specific mortality risk [29, 58, 79], although one of these studies reported lower mortality risk for those who quit smoking compared to those who continued to smoke [29].
Smoking: Key Points
Overall, evidence from observational studies has consistently suggested that smoking after CRC diagnosis increases the risk of CRC-specific and all-cause mortality. It seems plausible that smoking cessation would improve survival outcomes in CRC survivors, although direct evidence is limited.
Body Fatness and Body Composition after CRC Diagnosis
This review first focusses on studies that assessed BMI at or after CRC diagnosis. Next, we discuss weight changes and lastly, we describe the results of studies which quantified visceral adipose tissue or skeletal muscle mass from CT images.
Body Mass Index
Eleven population-based studies [16, 26, 32, 33, 48, 49, 62,64,66], two studies from adjuvant chemotherapy trials [67, 68], and one study among metastatic patients [34] assessed the association of BMI at or after CRC diagnosis and CRC outcomes (Table 1). Furthermore, 21 additional studies in the adjuvant setting were included in a pooled analyses of patients enrolled in trials of adjuvant chemotherapy [69]. Moreover, an additional article with pooled analyses in the metastatic setting included data of 25 treatment trials [70].
For underweight (either BMI < 18.5 or 20 kg/m2), all population-based studies [16, 26, 32, 33, 48, 62,64,66,63,65], the pooled analysis of studies in the adjuvant setting [69], and both publications in the metastatic setting [34, 70] reported higher all-cause mortality risk compared to normal weight individuals. The majority of these studies [26, 32, 34, 48, 62, 65, 69, 70] reported statistically significant results (Table 1). In the largest population-based study, ~ 3400 men and women diagnosed with stage I to III CRC from the Kaiser Permanente Northern California population, underweight at diagnosis was associated with a threefold increased all-cause mortality risk (HR 3.01; 95% CI 1.88–4.83) compared to normal weight [32]. However, most other studies report a 1.5- to 2-fold increased risk (Table 1). Generally, similar results were reported for CRC-specific mortality and cancer recurrence (Table 1).
For overweight (defined as BMI 25.0–24.9 kg/m2), all population-based studies [16, 26, 32, 33, 48, 49, 62, 64–66] reported lower all-cause mortality risk compared to normal weight individuals, of which three were statistically significant [48, 49, 62]. However, studies in the adjuvant setting of a chemotherapy trial reported that overweight individuals had a similar all-cause mortality risk as normal weight individuals (Table 1). For metastatic patients participating in treatment trials, all-cause mortality risk was lowest at BMI 28 kg/m2 [70], while overweight was associated with an increased all-cause mortality risk among a general population of patients diagnosed with metastatic disease (HR 1.23; 95% CI 1.03–1.46) [34]. Generally, similar results were reported for CRC-specific mortality and cancer recurrence (Table 1).
For obesity (BMI ≥ 30 kg/m2), none of the population-based studies [16, 26, 32, 33, 48, 49, 62, 64–66] reported statistically significant associations with all-cause mortality. Nevertheless, the only study (Kaiser Permanente Northern California cohort) that reported on a separate group with class II or III obesity (BMI ≥ 35 kg/m2) reported a statistically significant increased all-cause mortality risk [32]. Within the adjuvant setting pooled analyses showed a modest increased all-cause mortality risk (HR 1.10; 95% CI 1.04–1.17) compared with normal weight [69]. Within the metastatic setting, both publications showed that obese individuals had a somewhat similar, or lower, all-cause mortality risk as normal weight individuals [34, 70]. Generally, similar results were reported for CRC-specific mortality and cancer recurrence (Table 1).
Changes in Weight
Four studies [48, 80–82] reported on weight changes (Table 2). Two studies were population-based studies, a cohort from the Kaiser Permanente Northern California population [80] and an Australian cohort [48], and two studies were in the adjuvant setting, CALGB 89803 [81] and a cohort from the British Columbia Cancer Agency [82].
Large post-diagnosis weight loss (> 5 kg or ≥ 10%) was associated with a threefold increased all-cause and CRC mortality risk compared with stable weight in both population-based studies [48, 80]. Modest weight loss (2–4.9 kg or 5–9.9%) was also associated with increased all-cause and CRC mortality risk [48, 80], although only statistically significant in the Kaiser Permanente Northern California cohort [80]. In fact, the association between weight loss and mortality was present regardless of at-diagnosis BMI [80]. Large weight loss during adjuvant chemotherapy was associated with increased all-cause mortality and recurrence risk in a cohort from the British Columbia Cancer Agency [82], but not in CALGB 89803 [81].
Post-diagnosis weight gain was not associated with increased all-cause or CRC-specific mortality risk [48, 80, 81] or colon cancer recurrence [81, 82]. Furthermore, pre- to post-diagnosis weight loss or weight gain of >5 kg were both associated with a statistically significant 60% higher all-cause risk compared to stable weight [48].
Visceral Adipose Tissue
Three population-based studies [35, 39, 71], two studies in the adjuvant setting [72, 73], and one study among metastatic patients [74] reported on post-diagnosis visceral adipose tissue and all-cause mortality (Table 1). Most of these studies were small (n = 62 to 339), except for the population-based cohort from the Kaiser Permanente Northern California population (n ~ 3200) [39••].
For all-cause mortality, all population-based studies [35, 39••, 71] reported statistically non-significant associations with visceral adipose tissue (Table 1). Both studies among patients treated with chemotherapy [72, 73] reported an increased all-cause mortality risk with high visceral adipose tissue, of which one was statistically significant [72]. The study among metastatic CRC patients [74] reported a statistically significant increased all-cause mortality risk for high visceral adipose tissue among patients treated with chemotherapy plus the angiogenesis inhibitor bevacizumab, but not among patients treated with chemotherapy only.
Skeletal Muscle Mass
Four population-based studies [35, 38, 39••, 75], one study in the adjuvant setting [37] and three studies among patients with metastatic disease [36, 76, 77] reported on all-cause mortality (Table 1). Most of these studies were small (n = 67 to 339), except two population-based cohorts, from the Kaiser Permanente Northern California population (n ~ 3200) [39••] and from a single-institution hospital cohort that included stage I–IV patients [38].
Seven out of eight studies [36,37,38, 39••, 75,76,77] reported increased all-cause mortality risk for low skeletal muscle mass, of which five were statistically significant [37, 38, 39••, 75, 76] (Table 1). A meta-analysis, based on three small studies [75,76,77], concluded that a low muscle mass was statistically significantly associated with a more than twofold increased all-cause mortality risk (HR 2.25; 95% CI 1.63–3.09) [20]. The only large population-based cohort with non-metastatic patients, from Kaiser Permanente Northern California, showed an almost 30% increased risk of overall mortality and 50% increased risk of CRC-specific mortality [39••].
One study among metastatic patients reported on loss of muscle mass during chemotherapy [36]. This study showed that ≥ 9% loss of muscle mass during chemotherapy was associated with a more than fourfold increased all-cause mortality risk (HR 4.47; 95% CI 2.21–9.05) [36].
Body Fatness and Body Composition: Key Points
Body fatness was studied most often by assessment of body mass index, while only few studies assessed other measures of body composition. Altogether, the results of studies across the three study categories (population-based, adjuvant, and metastatic setting) suggest a J- or L-shaped association between BMI and all-cause mortality or CRC-specific mortality risk. The risk of death was highest among patients who were underweight, while lowest risk was seen in patients with a BMI between 25 and < 30 kg/m2. If obesity confers an additional mortality risk compared to normal weight or overweight patients remains uncertain. Nevertheless, the most recent meta-analysis of post-diagnosis BMI concluded that obesity was statistically significantly associated with a modest 8% increased all-cause mortality risk (HR 1.08; 95% CI 1.03–1.13) compared to normal weight, while no association was found between obesity and CRC-specific mortality [17••]. Weight loss in the first 2 years after diagnosis was consistently associated with increased mortality risk and this association was independent of BMI at CRC diagnosis. Currently, there are no intentional weight loss trials among CRC survivors that assessed mortality risk [86••] and no study that assessed the effect of weight loss after treatment was successfully completed. That being overweight, and in some studies even obese states, seem to be associated with improved survival compared to normal weight is called the “obesity paradox.” The obesity paradox could be explained by several methodological issues, including the crudeness of BMI as a measure of body fatness, especially in a cancer patient population where loss of weight and lean body mass is a strong adverse factor [87].
Other measures used to study the association between body composition and CRC outcomes were visceral adipose tissue and muscle mass quantified from CT images; studies with other measures, such as waist circumference, are currently lacking. There is only limited evidence that visceral adiposity increased mortality risk. Across study categories, studies had mixed results. Only in the adjuvant setting, two small studies consistently showed increased all-cause mortality risk with higher visceral adipose tissue. Even though quantification of adipose tissue from CT scans is regarded as a more precise measure of adiposity than BMI, the usefulness of single-slice analysis might be limited [88]. On the other hand, evidence consistently shows that low muscle mass is associated with reduced survival, although each study used other cut points to define low muscle mass. The notion that the association between overweight and lower mortality is due solely to methodologic biases is refuted by results from the only large population-based study among non-metastatic CRC patients with available data for both BMI and body composition [39••]. Within the overweight BMI range between 25 and < 30 kg/m2, body composition appeared to explain why a BMI higher than normal is associated with the lowest mortality. The majority (78%) of patients in the overweight group had adequate muscle mass, while less than half (43%) of the patients with a normal BMI had adequate muscle mass. Furthermore, the obesity paradox could also be explained by clinical issues [87], such as metabolic health. One study at Kaiser Permanente investigated the combination of obesity and metabolic health and concluded that mortality risk was statistically significantly increased in obese patients with the metabolic syndrome, but not in metabolically healthy obese patients, compared with metabolically healthy non-obese patients [89].
Conclusions and Future Directions
In conclusion, this review suggests that some, albeit not all, modifiable risk factors for cancer incidence might also be associated with mortality risk after CRC diagnosis. CRC prognosis appears to be worse with increased physical inactivity, smoking, or being underweight after CRC diagnosis. Emerging evidence suggests that diets associated with a positive energy balance, e.g., high consumption of sugar-sweetened beverages, may negatively impact survival in CRC survivors. Nonetheless, data relating post-diagnosis diet to CRC prognosis are scarce; with less than three observational studies that have examined associations for each dietary pattern or individual food after CRC diagnosis. In contrast, high red and processed meat or alcohol intake, established risk factors for incident CRC, do not appear to be associated with mortality after CRC diagnosis. Whether overweight and obesity after CRC diagnosis might confer an additional mortality risk compared to normal weight is still controversial and might depend on how body fatness is assessed and whether muscle mass was accounted for.
Since the first review on lifestyle factors in CRC survivors in 2010 [90], many new studies in this evolving area of research were published and summarized in subsequent reviews and meta-analyses. This is the first paper to comprehensively review post-diagnosis diet, physical activity, smoking, and body composition together in one review. Our findings were generally consistent with previous work, regarding diet [4••], physical activity [7,8,9,10, 11••], smoking [13••], and underweight [16, 17••, 19], although we included new publications. Overweight, assessed by BMI, was consistently associated with lowest mortality risk, although discussion remains about the causal claims regarding the effects of BMI on post-diagnosis mortality for CRC survivors. The only large population-based study among non-metastatic CRC patients concluded that body composition, i.e., muscle mass, appeared to explain why a BMI higher than normal is associated with the lowest mortality risk [39••]. Moreover, low muscle mass was consistently associated with increased mortality risk. Besides observational data, there were no reported randomized controlled trials in smoking or alcohol cessation/reduction, while physical activity and/or dietary/excess weight interventions only reported on short-term outcomes [86••]. Only one small randomized trial assessed long-term follow-up among CRC survivors, finding significantly improved cancer-specific survival after dietary counseling [78].
As people do not have isolated behaviors, a multidimensional lifestyle approach would be most informative for exploring mortality risk and cancer recurrence, as well as for translating these findings into meaningful strategies to improve disease prognosis. Some randomized controlled trials with both dietary and physical activity components have included CRC survivors, but they usually did not test the impact of comprehensive lifestyle interventions on risk of cancer recurrence or survival [86••]. Furthermore, only one observational study evaluated the association of post-diagnosis comprehensive lifestyle patterns and CRC outcomes [91]. That study concluded that adherence to the WCRF recommendations on diet, physical activity, and body fatness was not statistically significantly associated with mortality [91]. However, lifestyle was assessed on average 9 years after diagnosis and survivors were therefore at low risk to die from CRC during subsequent follow-up. Further research on post-diagnosis lifestyle patterns is needed to understand the multifactorial nature of risk of mortality and cancer recurrence and, furthermore, to avoid overemphasis of single lifestyle factors.
The existing studies have several limitations. Few observational studies have reported on the association between post-diagnostic lifestyle and CRC outcomes adjusting for pre-diagnostic lifestyle; thus, it is unknown whether the observed associations between post-diagnostic lifestyle and survival are independent of pre-diagnosis lifestyle. Furthermore, only few studies assessed changes in lifestyle over time in relation to CRC outcomes, with weight change and smoking cessation studied most often. Large prospective cohort studies, such as NHS I, HPFS, the COLON study [92], and others [93, 94], provide further opportunities to examine post-diagnosis lifestyle changes in relation to CRC prognosis during different phases of the cancer trajectory.
Studies evaluating lifestyle factors and CRC outcomes mainly focused on mortality, while cancer recurrence and comorbidities are other important outcomes. Disease recurrence was usually reported by studies in the adjuvant setting, but is not commonly reported by population-based studies. Furthermore, definitions of recurrence were inconsistent between studies. Using the standard definitions proposed by Punt et al. [95] may add to the cross-comparability of future studies. In addition, few studies among CRC survivors studied incidence and progression of comorbidities, although some studies included cardiovascular-mortality as an endpoint. Only one study assessed the incidence of comorbidities after CRC diagnosis [96]. This study observed that BMI and sedentary behavior at 5 months post-diagnosis were associated with the development of comorbid cardiovascular disease in the first 3 years after CRC diagnosis.
More research is needed on the mechanisms underlying the impact of lifestyle after CRC diagnosis on prognosis. A lifestyle contributing to a positive energy balance and hyperinsulinemia has been suggested to be implicated in the prognosis of CRC [5, 97]. For instance, determinants of hyperinsulinemia, such as physical inactivity, excessive sedentary behavior, and several aspects of diet, are associated with increased mortality risk. The dietary factors included in this review that might be linked to insulin-related pathways, a Western dietary pattern [23, 42], sugar-sweetened beverages [23, 43], low coffee consumption [25], and higher dietary glycemic load [45] all showed increased mortality risk. Also, a high-insulinogenic diet [98] has been associated with increased mortality risk. However, these studies were almost all conducted in the same cohort embedded in a trial of adjuvant chemotherapy (CALGB 89803) [25, 42, 43, 45].
Overall, evidence is emerging that modifiable lifestyle factors after CRC diagnosis, such as physical activity, smoking, body composition, and diet could impact survival. Although, not all modifiable risk factors for cancer presentation seem relevant for cancer survivors. With increasing CRC survivorship, however, CRC recurrence should be studied as a key outcome within population-based studies of CRC survivors. Additionally, studies are needed to evaluate the development and progression of comorbidities after CRC diagnosis. Studying lifestyle patterns over time, by including multiple lifestyle factors simultaneously at different time points during the cancer trajectory, would lead to a greater understanding of the multifactorial influence on CRC prognosis. Additional data from prospective observational studies and randomized controlled trials are urgently needed and, ultimately, will allow for lifestyle recommendations that are specifically tailored to cancer survivors.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
World Cancer Research Fund/American Institute for Cancer Research. Continuous update project report. Food, nutrition, physical activity, and the prevention of colorectal cancer. 2011. www.wcrf.org/sites/default/files/Colorectal-Cancer-2011-Report.pdf.
Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention. CA Cancer J Clin. 2012;62(1):30–67. https://doi.org/10.3322/caac.20140.
World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR; 2007.
•• Van Blarigan EL, Meyerhardt JA. Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol. 2015. https://doi.org/10.1200/jco.2014.59.7799. This review summarizes the evidence regarding physical activity and diet after CRC diagnosis in relation to quality of life, disease recurrence, and survival.
Lee J, Jeon JY, Meyerhardt JA. Diet and lifestyle in survivors of colorectal cancer. Hematol Oncol Clin North Am. 2015;29(1):1–27. https://doi.org/10.1016/j.hoc.2014.09.005.
Schwedhelm C, Boeing H, Hoffmann G, Aleksandrova K, Schwingshackl L. Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr Rev. 2016;74(12):737–48. https://doi.org/10.1093/nutrit/nuw045.
Des Guetz G, Uzzan B, Bouillet T, Nicolas P, Chouahnia K, Zelek L, et al. Impact of physical activity on cancer-specific and overall survival of patients with colorectal cancer. Gastroenterol Res Pract. 2013;2013:340851. https://doi.org/10.1155/2013/340851.
Je Y, Jeon JY, Giovannucci EL, Meyerhardt JA. Association between physical activity and mortality in colorectal cancer: a meta-analysis of prospective cohort studies. International Journal of Cancer Journal international du cancer. 2013;133(8):1905–13. https://doi.org/10.1002/ijc.28208.
Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014. https://doi.org/10.1093/annonc/mdu012.
Wu S, Liu J, Wang X, Li M, Gan Y, Tang Y. Association of obesity and overweight with overall survival in colorectal cancer patients: a meta-analysis of 29 studies. Cancer causes & control: CCC. 2014;25(11):1489–502. https://doi.org/10.1007/s10552-014-0450-y.
•• Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical activity and cancer outcomes: a precision medicine approach. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(19):4766–75. https://doi.org/10.1158/1078-0432.ccr-16-0067. This review summarizes the epidemiologic literature relating postdiagnosis physical activity to cancer outcomes overall and by molecular/genetic subgroups.
Morales-Oyarvide V, Meyerhardt JA, Ng K. Vitamin D and physical activity in patients with colorectal cancer: epidemiological evidence and therapeutic implications. Cancer Journal (United States). 2016;22(3):223–31. https://doi.org/10.1097/ppo.0000000000000197.
•• Walter V, Jansen L, Hoffmeister M, Brenner H. Smoking and survival of colorectal cancer patients: systematic review and meta-analysis. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2014;25(8):1517–25. https://doi.org/10.1093/annonc/mdu040. This systematic review and meta-analysis provides a summary of the epidemiologic literature between smoking and survival of CRC patients, including all-cause mortality, CRC-specific mortality, disease-free survival, and recurrence-free survival.
Sharp L, McDevitt J, Brown C, Comber H. Smoking at diagnosis significantly decreases 5-year cancer-specific survival in a population-based cohort of 18 166 colon cancer patients. Aliment Pharmacol Ther. 2017;45(6):788–800. https://doi.org/10.1111/apt.13944.
Parkin E, O'Reilly DA, Sherlock DJ, Manoharan P, Renehan AG. Excess adiposity and survival in patients with colorectal cancer: a systematic review. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2014;15(5):434–51. https://doi.org/10.1111/obr.12140.
Schlesinger S, Siegert S, Koch M, Walter J, Heits N, Hinz S, et al. Postdiagnosis body mass index and risk of mortality in colorectal cancer survivors: a prospective study and meta-analysis. Cancer causes & control : CCC. 2014;25(10):1407–18. https://doi.org/10.1007/s10552-014-0435-x.
•• Lee J, Meyerhardt JA, Giovannucci E, Jeon JY. Association between body mass index and prognosis of colorectal cancer: a meta-analysis of prospective cohort studies. PLoS One. 2015;10(3):e0120706. https://doi.org/10.1371/journal.pone.0120706. This meta-analysis of prospective studies summarized the association of pre- and post-diagnostic BMI with CRC-specific mortality and all-cause mortality in patients with CRC.
Brown JC, Meyerhardt JA. Obesity and energy balance in GI cancer. J Clin Oncol. 2016;34(35):4217–24. https://doi.org/10.1200/jco.2016.66.8699.
Doleman B, Mills KT, Lim S, Zelhart MD, Gagliardi G. Body mass index and colorectal cancer prognosis: a systematic review and meta-analysis. Techniques in coloproctology. 2016;20(8):517–35. https://doi.org/10.1007/s10151-016-1498-3.
Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: a meta-analysis and systematic review. Eur J Cancer. 2016;57:58–67. https://doi.org/10.1016/j.ejca.2015.12.030.
Xiao J, Mazurak VC, Olobatuyi TA, Caan BJ, Prado CM. Visceral adiposity and cancer survival: a review of imaging studies. European journal of cancer care. 2016. https://doi.org/10.1111/ecc.12611.
Malietzis G, Aziz O, Bagnall NM, Johns N, Fearon KC, Jenkins JT. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2015;41(2):186–96. https://doi.org/10.1016/j.ejso.2014.10.056.
Fung TT, Kashambwa R, Sato K, Chiuve SE, Fuchs CS, Wu K, et al. Post diagnosis diet quality and colorectal cancer survival in women. PLoS One. 2014;9(12):e115377. https://doi.org/10.1371/journal.pone.0115377.
Ratjen I, Schafmayer C, di Giuseppe R, Waniek S, Plachta-Danielzik S, Koch M, et al. Postdiagnostic Mediterranean and healthy Nordic dietary patterns are inversely associated with all-cause mortality in long-term colorectal cancer survivors. J Nutr. 2017;147(4):636–44. https://doi.org/10.3945/jn.116.244129.
Guercio BJ, Sato K, Niedzwiecki D, Ye X, Saltz LB, Mayer RJ, et al. Coffee intake, recurrence, and mortality in stage III colon cancer: results from CALGB 89803 (alliance). J Clin Oncol. 2015. https://doi.org/10.1200/jco.2015.61.5062.
Tamakoshi A, Nakamura K, Ukawa S, Okada E, Hirata M, Nagai A, et al. Characteristics and prognosis of Japanese colorectal cancer patients: the BioBank Japan project. Journal of epidemiology. 2017;27(3):S36–42. https://doi.org/10.1016/j.je.2016.12.004.
Yang B, Gapstur SM, Newton CC, Jacobs EJ, Campbell PT. Alcohol intake and mortality among survivors of colorectal cancer: the Cancer Prevention Study II Nutrition Cohort. Cancer. 2017. https://doi.org/10.1002/cncr.30556.
Tao L, Wang R, Gao YT, Yuan JM. Impact of postdiagnosis smoking on long-term survival of cancer patients: the Shanghai Cohort Study. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(12):2404–11. https://doi.org/10.1158/1055-9965.epi-13-0805-t.
Yang B, Jacobs EJ, Gapstur SM, Stevens V, Campbell PT. Active smoking and mortality among colorectal cancer survivors: the Cancer Prevention Study II nutrition cohort. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(8):885–93. https://doi.org/10.1200/jco.2014.58.3831.
Sharp L, McDevitt J, Brown C, Carsin AE, Comber H. Association between smoking at diagnosis and cause-specific survival in patients with rectal cancer: results from a population-based analysis of 10,794 cases. Cancer. 2017. https://doi.org/10.1002/cncr.30583.
Rasouli MA, Moradi G, Roshani D, Nikkhoo B, Ghaderi E, Ghaytasi B. Prognostic factors and survival of colorectal cancer in Kurdistan province, Iran: a population-based study (2009-2014). Medicine. 2017;96(6):e5941. https://doi.org/10.1097/md.0000000000005941.
Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML, et al. Analysis of body mass index and mortality in patients with colorectal cancer using causal diagrams. JAMA Oncol. 2016;2(9):1137–45. https://doi.org/10.1001/jamaoncol.2016.0732.
Walter V, Jansen L, Hoffmeister M, Ulrich A, Roth W, Bläker H, et al. Prognostic relevance of prediagnostic weight loss and overweight at diagnosis in patients with colorectal cancer. Am J Clin Nutr. 2016;104(4):1110–20. https://doi.org/10.3945/ajcn.116.136531.
Patel GS, Ullah S, Beeke C, Hakendorf P, Padbury R, Price TJ, et al. Association of BMI with overall survival in patients with mCRC who received chemotherapy versus EGFR and VEGF-targeted therapies. Cancer Medicine. 2015;4(10):1461–71. https://doi.org/10.1002/cam4.490.
Black D, Mackay C, Ramsay G, Hamoodi Z, Nanthakumaran S, Park KGM, et al. Prognostic value of computed tomography: measured parameters of body composition in primary operable gastrointestinal cancers. Ann Surg Oncol. 2017:1–11. https://doi.org/10.1245/s10434-017-5829-z.
Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, et al. Loss of muscle mass during chemotherapy is predictive for poor survival of patients with metastatic colorectal cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(12):1339–44. https://doi.org/10.1200/JCO.2015.63.6043.
Jung HW, Kim JW, Kim JY, Kim SW, Yang HK, Lee JW, et al. Effect of muscle mass on toxicity and survival in patients with colon cancer undergoing adjuvant chemotherapy. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 2015;23(3):687–94. https://doi.org/10.1007/s00520-014-2418-6.
Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, et al. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 2016;103(5):572–80. https://doi.org/10.1002/bjs.10075.
•• Caan BJ, Meyerhardt JA, Kroenke CH, Alexeeff S, Xiao J, Weltzien E, et al. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS study). Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017. https://doi.org/10.1158/1055-9965.EPI-17-0200. This study among non-metastatic CRC patients concluded that body composition appeared to explain why a BMI higher than normal is associated with the lowest mortality risk and therefore they refute the notion that the association between overweight and lower mortality is solely due to methodologic biases.
McCullough ML, Gapstur SM, Shah R, Jacobs EJ, Campbell PT. Association between red and processed meat intake and mortality among colorectal cancer survivors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(22):2773–82. https://doi.org/10.1200/JCO.2013.49.1126.
Yang B, McCullough ML, Gapstur SM, Jacobs EJ, Bostick RM, Fedirko V, et al. Calcium, vitamin D, dairy products, and mortality among colorectal cancer survivors: the Cancer Prevention Study-II Nutrition Cohort. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(22):2335–43. https://doi.org/10.1200/JCO.2014.55.3024.
Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Hu FB, Mayer RJ, et al. Association of dietary patterns with cancer recurrence and survival in patients with stage III colon cancer. JAMA : the journal of the American Medical Association. 2007;298(7):754–64. https://doi.org/10.1001/jama.298.7.754.
Fuchs MA, Sato K, Niedzwiecki D, Ye X, Saltz LB, Mayer RJ, et al. Sugar-sweetened beverage intake and cancer recurrence and survival in CALGB 89803 (alliance). PLoS One. 2014;9(6):e99816. https://doi.org/10.1371/journal.pone.0099816.
Lochhead P, Nishihara R, Qian ZR, Mima K, Cao Y, Sukawa Y, et al. Postdiagnostic intake of one-carbon nutrients and alcohol in relation to colorectal cancer survival. Am J Clin Nutr. 2015;102(5):1134–41. https://doi.org/10.3945/ajcn.115.115162.
Meyerhardt JA, Sato K, Niedzwiecki D, Ye C, Saltz LB, Mayer RJ, et al. Dietary glycemic load and cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Natl Cancer Inst. 2012;104(22):1702–11. https://doi.org/10.1093/jnci/djs399.
Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(22):3527–34. https://doi.org/10.1200/JCO.2006.06.0855.
Meyerhardt JA, Giovannucci EL, Ogino S, Kirkner GJ, Chan AT, Willett W, et al. Physical activity and male colorectal cancer survival. Arch Intern Med. 2009;169(22):2102–8. https://doi.org/10.1001/archinternmed.2009.412.
Baade PD, Meng X, Youl PH, Aitken JF, Dunn J, Chambers SK. The impact of body mass index and physical activity on mortality among patients with colorectal cancer in Queensland, Australia. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(7):1410–20. https://doi.org/10.1158/1055-9965.EPI-11-0079.
Kuiper JG, Phipps AI, Neuhouser ML, Chlebowski RT, Thomson CA, Irwin ML, et al. Recreational physical activity, body mass index, and survival in women with colorectal cancer. Cancer causes & control: CCC. 2012;23(12):1939–48. https://doi.org/10.1007/s10552-012-0071-2.
Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876–85. https://doi.org/10.1200/jco.2012.45.9735.
Arem H, Pfeiffer RM, Engels EA, Alfano CM, Hollenbeck A, Park Y, et al. Pre- and postdiagnosis physical activity, television viewing, and mortality among patients with colorectal cancer in the National Institutes of Health-AARP diet and health study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(2):180–8. https://doi.org/10.1200/JCO.2014.58.1355.
Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(22):3535–41. https://doi.org/10.1200/JCO.2006.06.0863.
Cao Y, Meyerhardt JA, Chan AT, Wu K, Fuchs CS, Giovannucci EL. Television watching and colorectal cancer survival in men. Cancer Causes Control. 2015;26(10):1467–76. https://doi.org/10.1007/s10552-015-0645-x.
Jadallah F, McCall JL, van Rij AM. Recurrence and survival after potentially curative surgery for colorectal cancer. The New Zealand medical journal. 1999;112(1091):248–50.
Ali RA, Dooley C, Comber H, Newell J, Egan LJ. Clinical features, treatment, and survival of patients with colorectal cancer with or without inflammatory bowel disease. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2011;9(7):584-9.e1–2. https://doi.org/10.1016/j.cgh.2011.04.016.
Warren GW, Kasza KA, Reid ME, Cummings KM, Marshall JR. Smoking at diagnosis and survival in cancer patients. International journal of cancer Journal international du cancer. 2013;132(2):401–10. https://doi.org/10.1002/ijc.27617.
Amri R, Bordeianou LG, Sylla P, Berger DL. Does active smoking induce hematogenous metastatic spread in colon cancer? Am J Surg. 2015;210(5):930–2. https://doi.org/10.1016/j.amjsurg.2015.03.034.
Walter V, Jansen L, Hoffmeister M, Ulrich A, Chang-Claude J, Brenner H. Smoking and survival of colorectal cancer patients: population-based study from Germany. International journal of cancer Journal international du cancer. 2015;137(6):1433–45. https://doi.org/10.1002/ijc.29511.
Munro AJ, Bentley AH, Ackland C, Boyle PJ. Smoking compromises cause-specific survival in patients with operable colorectal cancer. Clin Oncol (R Coll Radiol). 2006;18(6):436–40.
McCleary NJ, Niedzwiecki D, Hollis D, Saltz LB, Schaefer P, Whittom R, et al. Impact of smoking on patients with stage III colon cancer: results from cancer and leukemia group B 89803. Cancer. 2010;116(4):957–66. https://doi.org/10.1002/cncr.24866.
Phipps AI, Shi Q, Newcomb PA, Nelson GD, Sargent DJ, Alberts SR, et al. Associations between cigarette smoking status and colon cancer prognosis among participants in north central cancer treatment group phase III trial N0147. J Clin Oncol. 2013;31(16):2016–23. https://doi.org/10.1200/JCO.2012.46.2457.
Asghari-Jafarabadi M, Hajizadeh E, Kazemnejad A, Fatemi SR. Site-specific evaluation of prognostic factors on survival in Iranian colorectal cancer patients: a competing risks survival analysis. Asian Pacific journal of cancer prevention : APJCP. 2009;10(5):815–21.
Hines RB, Shanmugam C, Waterbor JW, McGwin G Jr, Funkhouser E, Coffey CS, et al. Effect of comorbidity and body mass index on the survival of African-American and Caucasian patients with colon cancer. Cancer. 2009;115(24):5798–806. https://doi.org/10.1002/cncr.24598.
Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of body mass index on survival after colorectal cancer diagnosis: the cancer prevention study-II nutrition cohort. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(1):42–52. https://doi.org/10.1200/JCO.2011.38.0287.
Chin CC, Kuo YH, Yeh CY, Chen JS, Tang R, Changchien CR, et al. Role of body mass index in colon cancer patients in Taiwan. World journal of gastroenterology: WJG. 2012;18(31):4191–8. https://doi.org/10.3748/wjg.v18.i31.4191.
Alipour S, Kennecke HF, Woods R, Lim HJ, Speers C, Brown CJ, et al. Body mass index and body surface area and their associations with outcomes in stage II and III colon cancer. J Gastrointest Cancer. 2013;44(2):203–10. https://doi.org/10.1007/s12029-012-9472-4.
Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Benson AB 3rd, Macdonald JS, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98(3):484–95. https://doi.org/10.1002/cncr.11544.
Meyerhardt JA, Tepper JE, Niedzwiecki D, Hollis DR, McCollum AD, Brady D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from intergroup trial 0114. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22(4):648–57. https://doi.org/10.1200/JCO.2004.07.121.
Sinicrope FA, Foster NR, Yothers G, Benson A, Seitz JF, Labianca R, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119(8):1528–36. https://doi.org/10.1002/cncr.27938.
Renfro LA, Loupakis F, Adams RA, Seymour MT, Heinemann V, Schmoll HJ, et al. Body mass index is prognostic in metastatic colorectal cancer: pooled analysis of patients from first-line clinical trials in the ARCAD database. J Clin Oncol. 2016;34(2):144–50. https://doi.org/10.1200/jco.2015.61.6441.
Rickles AS, Iannuzzi JC, Mironov O, Deeb AP, Sharma A, Fleming FJ, et al. Visceral obesity and colorectal cancer: are we missing the boat with BMI? Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2013;17(1):133–143; discussion p 43. https://doi.org/10.1007/s11605-012-2045-9.
Clark W, Siegel EM, Chen YA, Zhao X, Parsons CM, Hernandez JM, et al. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg. 2013;216(6):1070–81. https://doi.org/10.1016/j.jamcollsurg.2013.01.007.
Lee CS, Murphy DJ, McMahon C, Nolan B, Cullen G, Mulcahy H, et al. Visceral adiposity is a risk factor for poor prognosis in colorectal cancer patients receiving adjuvant chemotherapy. Journal of Gastrointestinal Cancer. 2015;46(3):243–50. https://doi.org/10.1007/s12029-015-9709-0.
Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil JP, et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59(3):341–7. https://doi.org/10.1136/gut.2009.188946.
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, et al. Sarcopenia is a negative prognostic factor after curative resection of colorectal cancer. Ann Surg Oncol. 2015;22(8):2663–8. https://doi.org/10.1245/s10434-014-4281-6.
van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99(4):550–7. https://doi.org/10.1002/bjs.7823.
Thoresen L, Frykholm G, Lydersen S, Ulveland H, Baracos V, Prado CM, et al. Nutritional status, cachexia and survival in patients with advanced colorectal carcinoma. Different assessment criteria for nutritional status provide unequal results. Clin Nutr. 2013;32(1):65–72. https://doi.org/10.1016/j.clnu.2012.05.009.
Ravasco P, Monteiro-Grillo I, Camilo M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: long-term follow-up of a randomized controlled trial of nutritional therapy. Am J Clin Nutr. 2012;96(6):1346–53. https://doi.org/10.3945/ajcn.111.018838.
Phipps AI, Baron J, Newcomb PA. Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival: the Seattle colon cancer family registry. Cancer. 2011;117(21):4948–57. https://doi.org/10.1002/cncr.26114.
Meyerhardt JA, Kroenke CH, Prado CM, Kwan ML, Castillo A, Weltzien E, et al. Association of Weight Change after colorectal cancer diagnosis and outcomes in the Kaiser Permanente northern California population. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2016;26(1):30–7. https://doi.org/10.1158/1055-9965.EPI-16-0145.
Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Nelson H, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from cancer and leukemia group B 89803. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(25):4109–15. https://doi.org/10.1200/JCO.2007.15.6687.
Vergidis J, Gresham G, Lim HJ, Renouf DJ, Kennecke HF, Ruan JY, et al. Impact of weight changes after the diagnosis of stage III colon cancer on survival outcomes. Clin Colorectal Cancer. 2016;15(1):16–23. https://doi.org/10.1016/j.clcc.2015.07.002.
Courneya KS, Booth CM, Gill S, O'Brien P, Vardy J, Friedenreich CM, et al. The colon health and life-long exercise change trial: a randomized trial of the National Cancer Institute of Canada clinical trials group. Curr Oncol. 2008;15(6):279–85.
Australian Gastro-Intestinal Trials Group. CHALLENGE clinical trial. https://agitg.org.au/challenge-clinical-trial/. 2017. Accessed 7 August 2017.
Courneya KS, Vardy JL, O'Callaghan CJ, Friedenreich CM, Campbell KL, Prapavessis H, et al. Effects of a structured exercise program on physical activity and fitness in colon cancer survivors: one year feasibility results from the CHALLENGE trial. Cancer Epidemiol Biomark Prev. 2016;25(6):969–77. https://doi.org/10.1158/1055-9965.epi-15-1267.
•• Moug SJ, Bryce A, Mutrie N, Anderson AS. Lifestyle interventions are feasible in patients with colorectal cancer with potential short-term health benefits: a systematic review. Int J Color Dis. 2017;32(6):765–75. https://doi.org/10.1007/s00384-017-2797-5. This systematic review assessed the evidence for the feasibility of performing lifestyle interventions in CRC patients and evaluated any short- and long-term health benefits.
Lennon H, Sperrin M, Badrick E, Renehan AG. The obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18(9):56. https://doi.org/10.1007/s11912-016-0539-4.
Shuster A, Patlas M, Pinthus JH, Mourtzakis M. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85(1009):1–10. https://doi.org/10.1259/bjr/38447238.
Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Dannenberg AJ, et al. Metabolic dysfunction, obesity, and survival among patients with early-stage colorectal cancer. J Clin Oncol. 2016;34(30):3664–71. https://doi.org/10.1200/jco.2016.67.4473.
Vrieling A, Kampman E. The role of body mass index, physical activity, and diet in colorectal cancer recurrence and survival: a review of the literature. Am J Clin Nutr. 2010;92(3):471–90. https://doi.org/10.3945/ajcn.2010.29005.
Inoue-Choi M, Robien K, Lazovich D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22(5):792–802. https://doi.org/10.1158/1055-9965.EPI-13-0054.
Winkels RM, Heine-Broring RC, van Zutphen M, van Harten-Gerritsen S, Kok DE, van Duijnhoven FJ, et al. The COLON study: colorectal cancer: longitudinal, observational study on nutritional and lifestyle factors that may influence colorectal tumour recurrence, survival and quality of life. BMC Cancer. 2014;14(1):374. https://doi.org/10.1186/1471-2407-14-374.
Soares-Miranda L, Abreu S, Silva M, Peixoto A, Ramalho R, da Silva PC, et al. Cancer survivor study (CASUS) on colorectal patients: longitudinal study on physical activity, fitness, nutrition, and its influences on quality of life, disease recurrence, and survival. Rationale and design. Int J Color Dis. 2016. https://doi.org/10.1007/s00384-016-2671-x.
van Roekel EH, Bours MJL, de Brouwer CPM, Ten Napel H, Sanduleanu S, Beets GL, et al. The applicability of the international classification of functioning, disability, and health to study lifestyle and quality of life of colorectal cancer survivors. Cancer Epidemiol Biomark Prev. 2014;23(7):1394–405. https://doi.org/10.1158/1055-9965.epi-13-1144.
Punt CJA, Buyse M, Köhne C-H, Hohenberger P, Labianca R, Schmoll HJ, et al. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst. 2007;99(13):998–1003. https://doi.org/10.1093/jnci/djm024.
Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co-morbid cardiovascular disease in a population-based cohort of colorectal cancer survivors. Eur J Cancer. 2011;47(2):267–76. https://doi.org/10.1016/j.ejca.2010.10.002.
Vigneri PG, Tirro E, Pennisi MS, Massimino M, Stella S, Romano C, et al. The insulin/IGF system in colorectal cancer development and resistance to therapy. Front Oncol. 2015;5:230. https://doi.org/10.3389/fonc.2015.00230.
Keum N, Yuan C, Nishihara R, Zoltick E, Hamada T, Martinez Fernandez A, et al. Dietary glycemic and insulin scores and colorectal cancer survival by tumor molecular biomarkers. Int J Cancer. 2017;140(12):2648–56. https://doi.org/10.1002/ijc.30683.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Moniek van Zutphen has received research support through a grant from the Dutch Cancer Society. Ellen Kampman declares that she has no conflict of interest. Edward L. Giovannucci declares that he has no conflict of interest. Fränzel J.B. van Duijnhoven declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Nutrition and Nutritional Interventions in Colorectal Cancer
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van Zutphen, M., Kampman, E., Giovannucci, E.L. et al. Lifestyle after Colorectal Cancer Diagnosis in Relation to Survival and Recurrence: A Review of the Literature. Curr Colorectal Cancer Rep 13, 370–401 (2017). https://doi.org/10.1007/s11888-017-0386-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-017-0386-1