Abstract
Purpose of Review
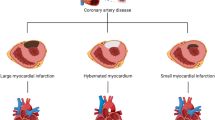
Collateral arteries create artery-artery anastomoses that could serve as natural bypasses that in the heart could relieve the various complications of ischemia heart disease. Recent work using animal models have begun to reveal details of how coronary collateral arteries form.
Recent Findings
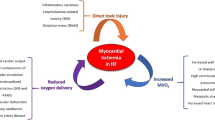
Mouse genetics has been used to study the cellular and molecular mechanisms of collateral artery development. Collateral arteries are not pre-existing in the mouse heart, and only form in response to injury. Myocardial infarction creates tissue hypoxia that triggers the expression of growth factors and chemokines that guide collaterogenesis. Collateral development is more robust in neonatal hearts when compared with adults, and contributes to neonatal heart regeneration.
Summary
The identification of signaling pathways and cellular responses underlying coronary collateral artery development suggests potential translational strategies. Continued investigation into this subject could lead to the identification of targetable pathways that induce collateral arteries for cardiac revascularization.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rocha SF, Adams RH. Molecular differentiation and specialization of vascular beds. Angiogenesis. 2009;12(2):139–47.
Blumgart HL, Schlesinger MJ, Zoll PM. Angina Pectoris, coronary failure and acute myocardial infarction. J Am Med Assoc. 1941;116(2):1–7.
Zoll PM, Wessler S, Schlesinger MJ. Interarterial coronary anastamoses in the human heart, with particular reference to anemia and relative cardiac anoxia. Circulation. 1951;IV:12.
Pohl T, Seiler C, Billinger M, Herren E, Wustmann K, Mehta H, et al. Frequency distribution of collateral flow and factors influencing collateral channel development. J Am Coll Cardiol. Elsevier Masson SAS. 2001;38(7):1872–8.
Wustmann K, Zbinden S, Windecker S, Meier B, Seiler C. Is there functional collateral flow during vascular occlusion in angiographically normal coronary arteries? Circulation. American Heart Association, Inc. 2003;107(17):2213–20.
Seiler C, Meier P. Historical aspects and relevance of the human coronary collateral circulation. Curr Cardiol Rev. 2014;10(1):2–16 PMCID: PMC3968590.
Schlesinger MJ. New radiopaque mass for vascular injection. Lab Investig. Lab Invest. 1957;6(1):1–11.
Seiler C, Stoller M, Pitt B, Meier P. The human coronary collateral circulation: development and clinical importance. Eur Heart J. 2013;34(34):2674–82.
Ylä-Herttuala S, Bridges C, Katz MG, Korpisalo P. Angiogenic gene therapy in cardiovascular diseases: dream or vision? Eur Heart J. 2017;11:ehw547.
Hulot J-S, Ishikawa K, Hajjar RJ. Gene therapy for the treatment of heart failure: promise postponed. Eur Heart J. 2016;37(21):1651–8.
Faber JE, Chilian WM, Deindl E, van Royen N, Simons M. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol. 2014;34(9):1854–9 American Heart Association, Inc PMCID: PMC4140974.
Hernandez GE, Iruela-Arispe ML. The many flavors of monocyte/macrophage--endothelial cell interactions. Curr Opin Hematol. 2020;27(3):181–9.
Maxwell MP, Hearse DJ, Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischaemia: a critical determinant of the rate of evolution and extent of myocardial infarction. Cardiovasc Res. 1987;21(10):737–46.
Zhang H, Faber JE. De-novo collateral formation following acute myocardial infarction: dependence on CCR2+ bone marrow cells. J Mol Cell Cardiol. 2015;87(C):4–16 Elsevier Ltd PMCID: PMC4637183.
•• Das S, Goldstone AB, Wang H, Farry J, D’Amato G, Paulsen MJ, et al. A unique collateral artery development program promotes neonatal heart regeneration. Cell. 2019;176(5):1128–1142.e18 PMCID: PMC6435282. Lineage tracing and mouse genetics demonstrated that collateral arteries are formed by artery cell migration, proliferation, and coalescence into collateral arteries. This process is driven by CXCL12/CXCR4 signaling and facilitates neonatal heart regeneration.
Faber JE, Storz JF, Cheviron ZA, Zhang H. High-altitude rodents have abundant collaterals that protect against tissue injury after cerebral, coronary and peripheral artery occlusion. J Cereb Blood Flow Metab. 6 ed. 2020;145(1):0271678X2094260–14.
Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Am Assoc Adv Sci. 2011;331(6020):1078–80 PMCID: PMC3099478.
Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci U S A. 2013;110(1):187–92 National Acad Sciences PMCID: PMC3538265.
Leid JM, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ. Primitive embryonic macrophages are required for coronary development and maturation. Circ Res. Lippincott Williams & Wilkins. 2016;118(10):1498–511.
He L, Liu Q, Hu T, Huang X, Zhang H, Tian X, et al. Genetic lineage tracing discloses arteriogenesis as the main mechanism for collateral growth in the mouse heart. Cardiovasc Res. 2016;109(3):419–30 PMCID: PMC4752045.
Döring Y, Pawig L, Weber C, Noels H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front Physiol. 2014;5:212 PMCID: PMC4052746.
Goldstone AB, Burnett CE, Cohen JE, Paulsen MJ, Eskandari A, Edwards BE, et al. SDF 1-alpha attenuates myocardial injury without altering the direct contribution of circulating cells. J Cardiovasc Transl Res. 2018;11(4):274–84.
Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, et al. Hypoxia induces heart regeneration in adult mice. Nature Nature Publishing Group. 2017;541(7636):222–7.
• Aghajanian A, Zhang H, Buckley BK, Wittchen ES, Ma WY, Faber JE. Decreased inspired oxygen stimulates de novo formation of coronary collaterals in adult heart. J Mol Cell Cardiol. 2021;150:1–11. Pre-conditioning mice in hypoxic conditions decreased infarcts induced by coronary ligation. Increased retrograde filling of coronary arteries suggests hypoxia induces collateral artery formation.
Toyota E, Warltier DC, Brock T, Ritman E, Kolz C, O’Malley P, et al. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005;112(14):2108–13.
Murthy SE, Dubin AE, Patapoutian A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol. Nature Publishing Group. 2017;18(12):771–83.
Chalothorn D, Faber JE. Formation and maturation of the native cerebral collateral circulation. J Mol Cell Cardiol. 2010;49(2):251–9 PMCID: PMC2885464.
Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. Nature Publishing Group. 2010;30(5):923–34.
Sealock R, Zhang H, Lucitti JL, Moore SM, Faber JE. Congenic fine-mapping identifies a major causal locus for variation in the native collateral circulation and ischemic injury in brain and lower extremity. Circ Res. 2014;114(4):660–71 PMCID: PMC3966023.
Cristofaro B, Shi Y, Faria M, Suchting S, Leroyer AS, Trindade A, et al. Dll4-Notch signaling determines the formation of native arterial collateral networks and arterial function in mouse ischemia models. Development. 2013;140(8):1720–9 Oxford University Press for The Company of Biologists Limited PMCID: PMC4074271.
Poduri A, Raftrey B, Chang AH, Rhee S, Van M, Red-Horse K. Endothelial cells respond to the direction of mechanical stimuli through SMAD signaling to regulate coronary artery size. Development. 2017;144(18):3241–52.
Sugden WW, Meissner R, Aegerter-Wilmsen T, Tsaryk R, Leonard EV, Bussmann J, et al. Endoglin controls blood vessel diameter through endothelial cell shape changes in response to haemodynamic cues. Nat Cell Biol. 2017;19(6):653–65.
Klems A, Rijssel J, Ramms AS, Wild R, Hammer J, Merkel M, et al. The GEF Trio controls endothelial cell size and arterial remodeling downstream of Vegf signaling in both zebrafish and cell models. Nat Commun. Springer US. 2020;14:1–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Regenerative Medicine
Rights and permissions
About this article
Cite this article
Red-Horse, K., Das, S. New Research Is Shining Light on How Collateral Arteries Form in the Heart: a Future Therapeutic Direction?. Curr Cardiol Rep 23, 30 (2021). https://doi.org/10.1007/s11886-021-01460-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11886-021-01460-z