Abstract
Purpose of Review
Despite major advances in terms of prevention, diagnosis, risk-stratification, management and rehabilitation, atherosclerosis and atherothrombosis continue to have major morbidity and mortality implications worldwide. Since the unraveling of the pivotal role of inflammation in atherothrombosis pathophysiology, several focused treatments have been proposed with the ultimate goal of preventing or treating myocardial infarction, stroke, and peripheral artery disease. In particular, given the centrality of interleukin-1 (IL-1), targeted anti-IL-1 agents have attracted substantial attention and efforts. Yet, uncertainty persists on the real risk-benefit and cost-benefit balance of anti-IL-1 agents in patients with or at risk of atherothrombosis.
Recent Findings
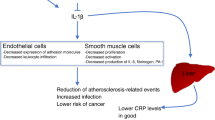
Several trials have been recently completed on atherothrombosis prevention and treatment with anti-IL-1 agents, ranging, for instance, from the large Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) trial to the series of translational studies conducted within the Virginia Commonwealth University-Anakinra Remodeling Trial (VCU-ART) platform. In light of the present scoping umbrella review, it appears evident that anti-IL-1 agents can reduce systemic inflammation and improve surrogate markers of cardiac and vascular function, with potential benefits on the risk of new/worsening heart failure. One trial suggested an increased risk of major adverse events with anti-interleukin-1 agents, possibly due to a rebound phenomenon, but this was based on a post-hoc analysis of a small number of events, and it was not supported by all other pertinent trials. The CANTOS study showed a potential hazard due to an increased risk of fatal infections, but the effect size was rather small. In addition, cost issues limit the foreseeable scope of these treatment strategies in unselected patients, calling instead for more refined prescribing.
Summary
The evidence base on the risk-benefit and cost-benefit profile of anti-IL-1 agents for atherothrombosis prevention and treatment has expanded substantially in the last decade. While largely dominated by the landmark CANTOS trial, effect estimates also including the VCU-ART trials suggest complex short- and long-term effects which may prove favorable in carefully selected patients with acute or chronically sustained inflammation. Conversely, more liberal use appears less promising, and further studies with currently available agents or novel ones are eagerly needed to better define their role in the era of precision molecular medicine.
Similar content being viewed by others
Change history
29 January 2020
The original version of this article unfortunately contained typo in the 2nd author’s family name. Instead of “Garmenda”, it should be “Garmendia”. The original version has been corrected.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Poston RN. Atherosclerosis: integration of its pathogenesis as a self-perpetuating propagating inflammation: a review. Cardiovasc Endocrinol Metab. 2019;8:51–61.
Touzani O, Boutin H, Chuquet J, Rothwell N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol. 1999;100:203–15.
Nguyen MT, Fernando S, Schwarz N, Tan JT, Bursill CA, Psaltis PJ. Inflammation as a therapeutic target in atherosclerosis. J Clin Med. 2019;8:E1109.
• Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol. 2003;41:1071–7 Comprehensive review on the interplay between inflammation, atherosclerosis, and thrombosis, with additional focus on diabetes mellitus.
Denes A, Pinteaux E, Rothwell NJ, Allan SM. Interleukin-1 and stroke: biomarker, harbinger of damage, and therapeutic target. Cerebrovasc Dis. 2011;32:517–27.
•• Abbate A, Van Tassell BW, Biondi-Zoccai GG. Blocking interleukin-1 as a novel therapeutic strategy for secondary prevention of cardiovascular events. BioDrugs. 2012;26:217–33 Comprehensive review on the pros and cons of anti-interleukin-1 agents for the prevention of cardiovascular disease.
Buckley LF, Abbate A. Interleukin-1 blockade in cardiovascular diseases: a clinical update. Eur Heart J. 2018;39:2063–9.
Ridker PM, Lüscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782–91.
Van Tassell BW, Raleigh JM, Abbate A. Targeting interleukin-1 in heart failure and inflammatory heart disease. Curr Heart Fail Rep. 2015;12:33–41.
•• Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–31 Pivotal randomized trial on the anti-interleukin-1 agent canakinumab for the secondary prevention of cardiovascular disease.
• Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol. 2010;105:1371–7.e1 First randomized trial from the Virginia Commonwealth University group on the anti-interleukin-1 agent anakirna for the treatment of patients with acute myocardial infarction.
Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra remodeling trial (2) (VCU-ART2) pilot study]. Am J Cardiol. 2013;111:1394–400.
Van Tassell BW, Lipinski MJ, Appleton D, Roberts CS, Kontos MC, Abouzaki N, et al. Rationale and design of the Virginia Commonwealth University-Anakinra Remodeling Trial-3 (VCU-ART3): a randomized, placebo-controlled, double-blinded, multicenter study. Clin Cardiol. 2018;41:1004–8.
Zhao TX, Mallat Z. Targeting the immune system in atherosclerosis: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1691–706.
•• Biondi-Zoccai G, editor. Umbrella reviews: evidence synthesis with overviews of reviews and meta-epidemiologic studies. Cham: Springer International Publishing; 2016. Comprehensive textbook highlighting the strengths and weaknesses of umbrella reviews for medical-decision making.
Oxman AD, Guyatt GH. Validation of an index of the quality of review articles. J Clin Epidemiol. 1991;44:1271–8.
Abbate A, Kontos MC, Abouzaki NA, Melchior RD, Thomas C, Van Tassell BW, et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol. 2015;115:288–92.
Buckley LF, Carbone S, Trankle CR, Canada JM, Erdle CO, Regan JA, et al. Effect of Interleukin-1 blockade on left ventricular systolic performance and work: a post hoc pooled analysis of 2 clinical trials. J Cardiovasc Pharmacol. 2018;72:68–70.
• Panahi M, Papanikolaou A, Torabi A, Zhang JG, Khan H, Vazir A, et al. Immunomodulatory interventions in myocardial infarction and heart failure: a systematic review of clinical trials and meta-analysis of IL-1 inhibition. Cardiovasc Res. 2018;114:1445–61.
Zheng ZH, Zeng X, Nie XY, Cheng YJ, Liu J, Lin XX, et al. Interleukin-1 blockade treatment decreasing cardiovascular risk. Clin Cardiol. 2019;42:942–51.
Cavalli G, Foppoli M, Cabrini L, Dinarello CA, Tresoldi M, Dagna L. Interleukin-1 receptor blockade rescues myocarditis-associated end-stage heart failure. Front Immunol. 2017;8:131.
Cavalli G, Pappalardo F, Mangieri A, Dinarello CA, Dagna L, Tresoldi M. Treating life-threatening myocarditis by blocking Interleukin-1. Crit Care Med. 2016;44:e751–4.
Choudhury RP, Birks JS, Mani V, Biasiolli L, Robson MD, L'Allier PL, et al. Arterial effects of Canakinumab in patients with atherosclerosis and type 2 diabetes or glucose intolerance. J Am Coll Cardiol. 2016;68:1769–80.
•• Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. Eur Heart J. 2015;36:377–84 Randomized trial on the anti-interleukin-1 agent anakinra for the treatment of patients with acute coronary syndromes.
Nowak KL, Chonchol M, Ikizler TA, Farmer-Bailey H, Salas N, Chaudhry R, et al. IL-1 inhibition and vascular function in CKD. J Am Soc Nephrol. 2017;28:971–80.
Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–48.
Smith CJ, Hulme S, Vail A, Heal C, Parry-Jones AR, Scarth S, et al. SCIL-STROKE (subcutaneous interleukin-1 receptor antagonist in ischemic stroke): a randomized controlled phase 2 trial. Stroke. 2018;49:1210–6.
Trankle CR, Canada JM, Cei L, Abouzaki N, Oddi-Erdle C, Kadariya D, et al. Usefulness of canakinumab to improve exercise capacity in patients with long-term systolic heart failure and elevated C-reactive protein. Am J Cardiol. 2018;122:1366–70.
Van Tassell BW, Abouzaki NA, Oddi Erdle C, Carbone S, Trankle CR, Melchior RD, et al. Interleukin-1 blockade in acute decompensated heart failure: a randomized, double-blinded, placebo-controlled pilot study. J Cardiovasc Pharmacol. 2016;67:544–51.
Van Tassell BW, Arena R, Biondi-Zoccai G, Canada JM, Oddi C, Abouzaki NA, et al. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardiol. 2014;113:321–7.
Van Tassell BW, Arena RA, Toldo S, Mezzaroma E, Azam T, Seropian IM, et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7:e33438.
• Van Tassell BW, Canada J, Carbone S, Trankle C, Buckley L, Oddi Erdle C, et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (Recently Decompensated Heart Failure Anakinra Response Trial). Circ Heart Fail. 2017;10:e004373 Pilot randomized trial highlighting the potential the anti-interleukin-1 agent anakinra for the treatment of heart failure.
Van Tassell BW, Trankle CR, Canada JM, Carbone S, Buckley L, Kadariya D, et al. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11:e005036.
Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139:1289–99.
Sehested TSG, Bjerre J, Ku S, Chang A, Jahansouz A, Owens DK, et al. Cost-effectiveness of canakinumab for prevention of recurrent cardiovascular events. JAMA Cardiol. 2019;4:128–35.
Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43.
Ridker PM. C-reactive protein, inflammation, and cardiovascular disease: clinical update. Tex Heart Inst J. 2005;32:384–6.
Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, et al. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol. 2018;72:2071–81.
Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–32.
Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–65.
Lobzhanidze G. Association between left ventricular ejection fraction and renal impairment in patients with cardio-renal syndrome type 2. Minerva Cardioangiol. 2018;66:520–1.
De Vecchis R, Cesaro A, Ariano C. Differential effects of the phosphodiesterase inhibition in chronic heart failure depending on the echocardiographic phenotype (HFREF or HFpEF): a meta-analysis. Minerva Cardioangiol. 2018;66:659–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Giuseppe Biondi-Zoccai, Cristian M. Garmendia, Arturo Giordano, Giacomo Frati, Sebastiano Sciarretta, Barbara Antonazzo, and Francesco Versaci declare that they have no conflict of interest. Antonio Abbate reports grants and personal fees from Kiniksa, Novartis, Olatec, and Swedish Orphan Biovitrum outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Guarantor
G.B.Z. is the guarantor of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Evidence-Based Medicine, Clinical Trials and Their Interpretations
Rights and permissions
About this article
Cite this article
Biondi-Zoccai, G., Garmendia, C.M., Abbate, A. et al. Atherothrombosis Prevention and Treatment with Anti-interleukin-1 Agents. Curr Atheroscler Rep 22, 4 (2020). https://doi.org/10.1007/s11883-020-0819-1
Published:
DOI: https://doi.org/10.1007/s11883-020-0819-1