Abstract
Purpose of Review
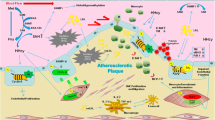
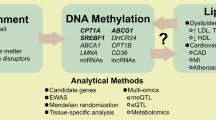
The quest for factors and mechanisms responsible for aberrant DNA methylation in human disease—including atherosclerosis—is a promising area of research. This review focuses on the role of fatty acids (FAs) as modulators of DNA methylation—in particular the role of mitochondrial beta-oxidation in FA-induced changes in DNA methylation during the progression of atherosclerosis.
Recent Findings
Recent publications have advanced the knowledge in all areas touched by this review: the causal role of lipids in shaping the DNA methylome, the associations between chronic degenerative disease and mitochondrial function, the lipid composition of the atheroma, and the relevance of DNA hypermethylation in atherosclerosis.
Summary
Evidence is beginning to emerge, linking the dynamics of FA type abundance, mitochondrial function, and DNA methylation in the atheroma and systemically. In particular, this review highlights mitochondrial beta-oxidation as an important regulator of DNA methylation in metabolic disease. Despite the many questions still unanswered, this area of research promises to identify mechanisms and molecular factors that establish a pathological gene expression pattern in atherosclerosis.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hotchkiss RD. The quantitative separation of purines, pyrimidines, and nucleosides by paper chromatography. J Biol Chem. 1948;175:315–32.
Suzuki MM, Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet. 2008;9:465–76.
Cooper DN, Taggart MH, Bird AP. Unmethylated domains in vertebrate DNA. Nucleic Acids Res. 1983;11:647–58.
Bird A, Taggart M, Frommer M, Miller OJ, Macleod D. A fraction of the mouse genome that is derived from islands of nonmethylated, CpG-rich DNA. Cell. 1985; 40:91–9.
Edwards JR, O’Donnell AH, Rollins RA, et al. Chromatin and sequence features that define the fine and gross structure of genomic methylation patterns. Genome Res. 2010;20:972–80.
Edwards JR, Yarychkivska O, Boulard M, Bestor TH. DNA methylation and DNA methyltransferases. Epigenetics Chromatin. 2017;10:23.
Zhang MQ, Ioshikhes IP. Large-scale human promoter mapping using CpG islands. Nat Genet. 2000;26:61–3.
Lister R, Pelizzola M, Dowen RH, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–22.
Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–92.
Bestor TH, Edwards JR, Boulard M. Notes on the role of dynamic DNA methylation in mammalian development. Proc Natl Acad Sci. 2015;112:6796–9.
Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W. Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philos Trans R Soc B Biol Sci. 2012;368:20110330.
Tang WWC, Kobayashi T, Irie N, Dietmann S, Surani MA. Specification and epigenetic programming of the human germ line. Nat Rev Genet. 2016;17:585–600.
Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, et al. Germline DNA demethylation dynamics and imprint erasure through 5-hydroxymethylcytosine. Science. 2013;339:448–52.
Bestor TH. Activation of mammalian DNA methyltransferase by cleavage of a Zn binding regulatory domain. EMBO J. 1992;11:2611–7.
Pradhan S, Bacolla A, Wells RD, Roberts RJ. Recombinant human DNA (cytosine-5) methyltransferase. I. Expression, purification, and comparison of de novo and maintenance methylation. J Biol Chem. 1999;274:33002–10.
Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011;25:2436–52.
Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92.
Gama-Sosa MA, Slagel VA, Trewyn RW, Oxenhandler R, Kuo KC, Gehrke CW, et al. The 5-methylcytosine content of DNA from human tumors. Nucleic Acids Res. 1983;11:6883–94.
Zoghbi HY, Beaudet AL. Epigenetics and human disease. Cold Spring Harb Perspect Biol. 2016;8:a019497.
Feinberg AP, Irizarry RA, Fradin D, et al. Personalized epigenomic signatures that are stable over time and covary with body mass index. Sci Transl Med. 2010;2:49ra67.
Wang X, Zhu H, Snieder H, et al. Obesity related methylation changes in DNA of peripheral blood leukocytes. BMC Med. 2010;8:87.
Murphy SK, Yang H, Moylan CA, et al. Relationship between methylome and transcriptome in patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:1076–87.
Dayeh T, Volkov P, Salö S, et al. Genome-wide DNA methylation analysis of human pancreatic islets from type 2 diabetic and non-diabetic donors identifies candidate genes that influence insulin secretion. PLoS Genet. 2014;10:e1004160.
Zaina S, Heyn H, Carmona FJ, et al. DNA methylation map of human atherosclerosis. Circ Cardiovasc Genet. 2014;7:692–700.
• Paul DS, Teschendorff AE, Dang MANN, et al. Increased DNA methylation variability in type 1 diabetes across three immune effector cell types. Nat Commun. 2016;7:13555. This article highlights the importance of cell type on DNA methylation variability in the analysis of genome and gene-specific methylation profiles.
Hansen KD, Timp W, Bravo HC, et al. Increased methylation variation in epigenetic domains across cancer types. Nat Genet. 2011;43:768–75.
Xu X, Su S, Barnes VA, De Miguel C, Pollock J, Ownby D, et al. A genome-wide methylation study on obesity: differential variability and differential methylation. Epigenetics. 2013;8:522–33.
Córdova-Palomera A, Fatjó-Vilas M, Gastó C, Navarro V, Krebs M-O, Fañanás L. Genome-wide methylation study on depression: differential methylation and variable methylation in monozygotic twins. Transl Psychiatry. 2015;5:e557.
Lund G, Andersson L, Lauria M, Lindholm M, Fraga MF, Villar-Garea A, et al. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J Biol Chem. 2004;279:29147–54.
Valencia-Morales Mdel P, Zaina S, Heyn H, et al. The DNA methylation drift of the atherosclerotic aorta increases with lesion progression. BMC Med Genomics 2015;8:7.
Rakyan VK, Beyan H, Down TA, et al. Identification of type 1 diabetes-associated DNA methylation variable positions that precede disease diagnosis. PLoS Genet. 2011;7:e1002300.
Toperoff G, Aran D, Kark JD, et al. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet. 2012;21:371–83.
Bell JT, Tsai PC, Yang TP, et al. Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet. 2012;8:e1002629.
Turunen MP, Aavik E, Ylä-Herttuala S. Epigenetics and atherosclerosis. Biochim Biophys Acta. 2009;1790:886–91.
Sayols-Baixeras S, Irvin MR, Elosua R, Arnett DK, Aslibekyan SW. Epigenetics of lipid phenotypes. Curr Cardiovasc Risk Rep. 2016;10:31.
Houseman EA, Kelsey KT, Wiencke JK, Marsit CJ. Cell-composition effects in the analysis of DNA methylation array data: a mathematical perspective. BMC Bioinformatics. 2015;16:95.
Dunn J, Qiu H, Kim S, et al. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest. 2014;124:3187–99.
Cao Q, Wang X, Jia L, et al. Inhibiting DNA methylation by 5-Aza-2′-deoxycytidine ameliorates atherosclerosis through suppressing macrophage inflammation. Endocrinology. 2014;155:4925–38.
Yu J, Qiu Y, Yang J, Bian S, Chen G, Deng M, et al. DNMT1-PPARγ pathway in macrophages regulates chronic inflammation and atherosclerosis development in mice. Sci Rep. 2016;6:30053.
Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, et al. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation. 2013;128:2047–57.
Zaina S, Gonçalves I, Carmona FJ, Gomez A, Heyn H, Mollet IG, et al. DNA methylation dynamics in human carotid plaques after cerebrovascular events. Arterioscler Thromb Vasc Biol. 2015;35:1835–42.
Peeters W, Hellings WE, De Kleijn DP, De Vries JP, Moll FL, Vink A, et al. Carotid atherosclerotic plaques stabilize after stroke: insights into the natural process of atherosclerotic plaque stabilization. Arterioscler Thromb Vasc Biol. 2009;29:128–33.
Vaiserman A. Epidemiologic evidence for association between adverse environmental exposures in early life and epigenetic variation: a potential link to disease susceptibility? Clin Epigenetics. 2015;7:96.
Barrès R, Zierath JR. The role of diet and exercise in the transgenerational epigenetic landscape of T2DM. Nat Rev Endocrinol. 2016;12:441–51.
Zheng J, Xiao X, Zhang Q, Yu M. DNA methylation: the pivotal interaction between early-life nutrition and glucose metabolism in later life. Br J Nutr. 2014;112:1850–7.
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet (London, England). 1989;2:577–80.
Burdge GC, Hoile SP, Uller T, Thomas NA, Gluckman PD, Hanson MA, et al. Progressive, transgenerational changes in offspring phenotype and epigenotype following nutritional transition. PLoS One. 2011;6:e28282.
Öst A, Lempradl A, Casas E, et al. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell. 2014;159:1352–64.
Radford EJ, Ito M, Shi H, et al. In utero effects. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science. 2014;345:1255903.
Gaydos LJ, Wang W, Strome S. H3K27me and PRC2 transmit a memory of repression across generations and during development. Science. 2014;345:1515–8.
Strakovsky RS, Zhang X, Zhou D, Pan Y-X. The regulation of hepatic Pon1 by a maternal high-fat diet is gender specific and may occur through promoter histone modifications in neonatal rats. J Nutr Biochem. 2014;25:170–6.
Fernandez-Twinn DS, Alfaradhi MZ, Martin-Gronert MS, Duque-Guimaraes DE, Piekarz A, Ferland-McCollough D, et al. Downregulation of IRS-1 in adipose tissue of offspring of obese mice is programmed cell-autonomously through post-transcriptional mechanisms. Mol Metab. 2014;3:325–33.
Fullston T, Ohlsson Teague EMC, Palmer NO, DeBlasio MJ, Mitchell M, Corbett M, et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013;27:4226–43.
de Castro Barbosa T, Ingerslev LR, Alm PS, et al. High-fat diet reprograms the epigenome of rat spermatozoa and transgenerationally affects metabolism of the offspring. Mol Metab. 2016;5:184–97.
Zander-Fox DL, Fullston T, McPherson NO, Sandeman L, Kang WX, Good SB, et al. Reduction of mitochondrial function by FCCP during mouse cleavage stage embryo culture reduces birth weight and impairs the metabolic health of offspring1. Biol Reprod. 2015;92:124.
• Saben JL, Boudoures AL, Asghar Z, Thompson A, Drury A, Zhang W, et al. Maternal metabolic syndrome programs mitochondrial dysfunction via germline changes across three generations. Cell Rep. 2016;16:1–8. Highlights the important role of mitochondria in transgenerational inheritance of metabolic sydrome induced by high fat/high sucrose diet in mice.
•• Hou Y-J, Zhu C-C, Duan X, Liu H-L, Wang Q, Sun S-C. Both diet and gene mutation induced obesity affect oocyte quality in mice. Sci Rep. 2016;6:18858. Important evidence that the ovary exposed to high fat diet and displays DNA hypomethylation.
Zock PL, Blom WAM, Nettleton JA, Hornstra G. Progressing insights into the role of dietary fats in the prevention of cardiovascular disease. Curr Cardiol Rep. 2016;18:111.
Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–46.
Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk. Ann Intern Med. 2014;160:398–406.
de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978.
Harcombe Z, Baker JS, Cooper SM, Davies B, Sculthorpe N, DiNicolantonio JJ, et al. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Hear. 2015;2:e000196.
Ramsden CE, Zamora D, Majchrzak-Hong S, Faurot KR, Broste SK, Frantz RP, et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ. 2016;353:i1246.
Praagman J, Beulens JW, Alssema M, Zock PL, Wanders AJ, Sluijs I, et al. The association between dietary saturated fatty acids and ischemic heart disease depends on the type and source of fatty acid in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort. Am J Clin Nutr. 2016;103:356–65.
Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, et al. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am J Clin Nutr. 1999;70:1001–8.
Flores-Sierra J, Arredondo-Guerrero M, Cervantes-Paz B, Rodríguez-Ríos D, Alvarado-Caudillo Y, Nielsen FC, et al. The trans fatty acid elaidate affects the global DNA methylation profile of cultured cells and in vivo. Lipids Health Dis. 2016;15:75.
Barrès R, Osler ME, Yan J, Rune A, Fritz T, Caidahl K, et al. Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab. 2009;10:189–98.
Hall E, Volkov P, Dayeh T, Bacos K, Rönn T, Nitert MD, et al. Effects of palmitate on genome-wide mRNA expression and DNA methylation patterns in human pancreatic islets. BMC Med. 2014;12:103.
• Silva-Martínez GA, Rodríguez-Ríos D, Alvarado-Caudillo Y, et al. Arachidonic and oleic acid exert distinct effects on the DNA methylome. Epigenetics. 2016;11:321–34. An analysis of FA-specific effects on beta-oxidation-dependent DNA methylation.
Aslibekyan S, Wiener HW, Havel PJ, Stanhope KL, O’Brien DM, Hopkins SE, et al. DNA methylation patterns are associated with n-3 fatty acid intake in Yup’ik people. J Nutr. 2014; doi:10.3945/jn.113.187203.
Voisin S, Almén MS, Moschonis G, Chrousos GP, Manios Y, Schiöth HB. Dietary fat quality impacts genome-wide DNA methylation patterns in a cross-sectional study of Greek preadolescents. Eur J Hum Genet. 2015;23:654–62.
• de la Rocha C, Pérez-Mojica JE, Zenteno-De León S, et al. Associations between whole peripheral blood fatty acids and DNA methylation in humans. Sci Rep. 2016;6:25867. Details BMI-dependant associations between FA content and DNA methylation in metabolically healthy individuals.
Marchlewicz EH, Dolinoy DC, Tang L, et al. Lipid metabolism is associated with developmental epigenetic programming. Sci Rep. 2016;6:34857.
Tremblay BL, Guénard F, Rudkowska I, Lemieux S, Couture P, Vohl M-C. Epigenetic changes in blood leukocytes following an omega-3 fatty acid supplementation. Clin Epigenetics. 2017;9:43.
Perfilyev A, Dahlman I, Gillberg L, Rosqvist F, Iggman D, Volkov P, et al. Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: a randomized controlled trial. Am J Clin Nutr. 2017;105:991–1000.
• Ollikainen M, Ismail K, Gervin K, et al. Genome-wide blood DNA methylation alterations at regulatory elements and heterochromatic regions in monozygotic twins discordant for obesity and liver fat. Clin Epigenetics. 2015;7:39. Points to an association between fatty liver disease and blood methylation profiles in twins discordant for BMI and fatty liver.
McGarry JD, Mannaerts GP, Foster DW. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977;60:265–70.
•• Kirchner H, Sinha I, Gao H, et al. Altered DNA methylation of glycolytic and lipogenic genes in liver from obese and type 2 diabetic patients. Mol Metab. 2016;5:171–83. This study shows that hypomethylation is a characteristic of obese individuals before or at an early stage in the development of type 2 diabetes and shows that hypomethylation is associated with upregulation of glycolysis and de novo lipogenesis.
Erlinge D. Near-infrared spectroscopy for intracoronary detection of lipid-rich plaques to understand atherosclerotic plaque biology in man and guide clinical therapy. J Intern Med. 2015;278:110–25.
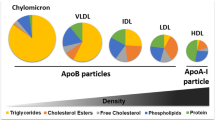
• Kolovou G, Kolovou V, Mavrogeni S. Lipidomics in vascular health: current perspectives. Vasc Health Risk Manag. 2015;11:333–42. A comprehensive view of the relevance of lipidomics for cardiovascular disease research.
• Ménégaut L, Masson D, Abello N, et al. Specific enrichment of 2-arachidonoyl-lysophosphatidylcholine in carotid atheroma plaque from type 2 diabetic patients. Atherosclerosis. 2016;251:339–47. A description of the atheroma lipidome.
Bojic LA, McLaren DG, Shah V, Previs SF, Johns DG, Castro-Perez JM. Lipidome of atherosclerotic plaques from hypercholesterolemic rabbits. Int J Mol Sci. 2014;15:23283–93.
•• Powell D, Gay J, Smith M, et al. Fatty acid desaturase 1 knockout mice are lean with improved glycemic control and decreased development of atheromatous plaque. Diabetes Metab Syndr Obes Targets Ther. 2016;9:185. By genetic manipulation, the work demonstrates the metabolic effects of the alteration of FA pool composition.
Varin A, Thomas C, Ishibashi M, et al. Liver X receptor activation promotes polyunsaturated fatty acid synthesis in macrophages: relevance in the context of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1357–65.
Yang Z-H, Gordon SM, Sviridov D, Wang S, Danner RL, Pryor M, et al. Dietary supplementation with long-chain monounsaturated fatty acid isomers decreases atherosclerosis and alters lipoprotein proteomes in LDLr −/− mice. Atherosclerosis. 2017;262:31–8.
Kamalakkannan S, Tirupathi Pichiah P, Kalaiselvi S, Arunachalam S, Achiraman S. Emu oil decreases atherogenic plaque formation in cafeteria diet-induced obese rats. J Sci Food Agric. 2016;96:3063–8.
Degirolamo C, Shelness GS, Rudel LL. LDL cholesteryl oleate as a predictor for atherosclerosis: evidence from human and animal studies on dietary fat. J Lipid Res. 2008;50:S434–9.
Kim J, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–14.
• Yu EP, Bennett MR. The role of mitochondrial DNA damage in the development of atherosclerosis. Free Radic Biol Med. 2016;100:223–30. Describes relevant advances in the field of mitochondrial biology and atherosclerosis.
• Yu J-W, Lee M-S. Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance. Arch Pharm Res. 2016;39:1503–18. Gathers up-to-date information on mitochondria-inflammasome functional interactions.
•• Dekkers KF, van Iterson M, Slieker RC, et al. Blood lipids influence DNA methylation in circulating cells. Genome Biol. 2016;17:138. A milestone that helps understanding the fundamental relationships between lipids and the DNA methylome.
Irvin MR, Zhi D, Joehanes R, et al. Epigenome-wide association study of fasting blood lipids in the genetics of lipid-lowering drugs and diet network study. Circulation. 2014;130:565–72.
Rangel-Salazar R, Wickström-Lindholm M, Aguilar-Salinas CA, et al. Human native lipoprotein-induced de novo DNA methylation is associated with repression of inflammatory genes in THP-1 macrophages. BMC Genomics. 2011;12:582.
• Vorkas PA, Shalhoub J, Lewis MR, Spagou K, Want EJ, Nicholson JK, et al. Metabolic phenotypes of carotid atherosclerotic plaques relate to stroke risk: an exploratory study. Eur J Vasc Endovasc Surg. 2016;52:5–10. An important description of metabolic markers of the carotid atheroma.
Garbin U, Baggio E, Stranieri C, et al. Expansion of necrotic core and shedding of Mertk receptor in human carotid plaques: a role for oxidized polyunsaturated fatty acids? Cardiovasc Res. 2013;97:125–33.
Bisgaard LS, Mogensen CK, Rosendahl A, Cucak H, Nielsen LB, Rasmussen SE, et al. Bone marrow-derived and peritoneal macrophages have different inflammatory response to oxLDL and M1/M2 marker expression—implications for atherosclerosis research. Sci Rep. 2016;6:35234.
• Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653–67. A must-read review by leading inflammation researchers.
Castillo-Díaz SA, Garay-Sevilla ME, Hernández-González MA, Solís-Martínez MO, Zaina S. Extensive demethylation of normally hypermethylated CpG islands occurs in human atherosclerotic arteries. Int J Mol Med. 2010;26:691–700.
Aavik E, Lumivuori H, Leppänen O, et al. Global DNA methylation analysis of human atherosclerotic plaques reveals extensive genomic hypomethylation and reactivation at imprinted locus 14q32 involving induction of a miRNA cluster. Eur Heart J. 2014;36:993–1000.
Sharma P, Kumar J, Garg G, Kumar A, Patowary A, Karthikeyan G, et al. Detection of altered global DNA methylation in coronary artery disease patients. DNA Cell Biol. 2008;27:357–65.
Volkmar M, Dedeurwaerder S, Cunha DA, et al. DNA methylation profiling identifies epigenetic dysregulation in pancreatic islets from type 2 diabetic patients. EMBO J. 2012;31:1405–26.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Acknowledgements
GL and SZ have received funding from the Kellogg’s Institute for Nutrition and Health, “Research Projects in Nutrition 2016” grant no. 100. Due to the extent of available literature and the multidisciplinary nature of this review, readers are in some instances referred to review articles. We apologize to all authors whose work was not directly cited.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Gertrud Lund and Silvio Zaina each declare no conflicts of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Genetics and Genomics
Rights and permissions
About this article
Cite this article
Zaina, S., Lund, G. Connecting the Dots Between Fatty Acids, Mitochondrial Function, and DNA Methylation in Atherosclerosis. Curr Atheroscler Rep 19, 36 (2017). https://doi.org/10.1007/s11883-017-0673-y
Published:
DOI: https://doi.org/10.1007/s11883-017-0673-y