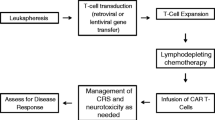
Opinion statement
Chimeric receptor antigen (CAR) T cells are an innovative cellular immunotherapeutic approach that involves genetic modification of T cells to express CAR targeting tumor antigen. Prior to the development of CAR-T, the only potential cure for patients with relapsed or refractory (RR) acute lymphoblastic leukemia (ALL) was allogeneic hematopoietic stem cell transplantation (HSCT). Several CAR-T cell products have been studied in prospective clinical trials which ultimately have resulted in the approval of one anti-CD19 CAR-T cell product in pediatric RR ALL: tisagenlecleucel (CD3ζ and 41BB). While some patients achieve durable responses with CAR-T, lack of response and relapse remains clinical challenges. Reasons for sub-optimal response include lack of CAR-T cell persistence and target antigen down-regulation. Future CARs are under development to improve long-term persistence and to be able to overcome resistance mechanisms associated with the disease and the hostile tumor microenvironment. With evolving understanding about CARs and new constructs under investigation, there is optimism that future products will improve the safety and efficacy from the current standard of care.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukemia. Lancet Oncol. 2013;14(6):e205–17.
Gokbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Huttmann A, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120(10):2032–41.
Thomas DA, Kantarjian H, Smith TL, Koller C, Cortes J, O’Brien S, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer. 1999;86(7):1216–30.
Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47.
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–53.
Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30(14):1663–9.
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–59.
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73.
Park JH, Geyer MB, Brentjens RJ. CD19-targeted CAR T cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127(26):3312–20.
Park JH, Brentjens RJ. Adoptive immunotherapy for B cell malignancies with autologous chimeric antigen receptor modified tumor targeted T cells. Discov Med. 2010;9(47):277–88.
Park JH, Brentjens RJ. Are all chimeric antigen receptors created equal? J Clin Oncol. 2015;33(6):651–3.
Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma. 1995;18(5–6):385–97.
•• Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–48 This is a pivotal, global study which led to approval of Tisagenlecleucel (anti-CD19 CAR-T cell) in relapsed refractory B cell acute lymphoblastic leukemia patients up to 25 years of age.
Erratum: Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. Blood. 2016;128(11):1533.
Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–15.
Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14(11):1264–70.
Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–6.
Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther: J Am Soc Gene Ther. 2009;17(8):1453–64.
Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106(9):3360–5.
Till BG, Jensen MC, Wang J, Qian X, Gopal AK, Maloney DG, et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood. 2012;119(17):3940–50.
Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202(7):907–12.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28.
Shah BD, Bishop MR, Oluwole OO, Logan A, Baer MR, Donnellan WB, et al. End of phase I results of ZUMA-3, a phase 1/2 study of KTE-X19, anti-CD19 chimeric antigen receptor (CAR) T cell therapy, in adult patients (pts) with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL). 2019;37(15_suppl):7006.
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–38.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–8.
• Schultz LM, Davis KL, Baggott C, Chaudry C, Marcy AC, Mavroukakis S, et al. Phase 1 study of CD19/CD22 bispecific chimeric antigen receptor (CAR) therapy in children and young adults with B cell acute lymphoblastic leukemia (ALL). Blood. 2018;132(Suppl 1):898 One of the reasons for treatment failure with CD19 CAR-T cell is down-regulation of CD19 receptor making CD19 CAR-T cells ineffective. This papers highlights novel multi-targeted (CD19/CD22) CAR-T cell to improve effectiveness of CAR-T cell in B cell malignancies.
Frey NV, Porter DL. Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2016;2016(1):567–72.
Schultz LM, Davis KL, Baggott C, Chaudry C, Marcy AC, Mavroukakis S, et al. Phase 1 Study of CD19/CD22 bispecific chimeric antigen receptor (CAR) therapy in children and young adults with B Cell Acute lymphoblastic leukemia (ALL). 2018;132(Suppl 1):898-.
•• Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release Syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–38 Initial clinical trials of CAR-T cell therapy use different grading system for CRS. Later, the American Society of Transplant and Cellular Therapy (ASTCT) developed a consensus grading system for CRS, outlined in this paper.
Nellan A, McCully CML, Cruz Garcia R, Jayaprakash N, Widemann BC, Lee DW, et al. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood. 2018;132(6):662–6.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17.
Park JH, Riviere I, Wang X, Bernal Y, Purdon T, Halton E, et al. Efficacy and safety of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed or refractory B-ALL. J Clin Oncol. 2015;33(15_suppl):7010.
Turtle CJ, Hanafi L-A, Berger C, Sommermeyer D, Pender B, Robinson EM, et al. Addition of fludarabine to cyclophosphamide lymphodepletion improves in vivo expansion of CD19 chimeric antigen receptor-modified T cells and clinical outcome in adults with B cell acute lymphoblastic leukemia. Blood. 2015;126(23):3773.
Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282–95.
Shah NN, Maatman T, Hari P, Johnson B. Multi-targeted CAR-T Cell therapies for B cell malignancies. Front Oncol. 2019;9:146.
Ghorashian S, Kramer AM, Onuoha S, Wright G, Bartram J, Richardson R, et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25(9):1408–14.
Lee DW III, Stetler-Stevenson M, Yuan CM, Shah NN, Delbrook C, Yates B, et al. Long-term outcomes following CD19 CAR T cell therapy for B-ALL are superior in patients receiving a fludarabine/cyclophosphamide preparative regimen and post-CAR hematopoietic stem cell transplantation. Blood. 2016;128(22):218.
• Shalabi H, Delbrook C, Stetler-Stevenson M, Yuan C, Steinberg SM, Yates B, et al. Chimeric antigen receptor T cell (CAR-T) therapy can render patients with ALL into PCR-negative remission and can be an effective bridge to transplant (HCT). Biol Blood Marrow Transplant. 2018;24(3):S25–S6 This retrospective analysis evaluated clinical outcome of patients who had allogeniec hematopoietic stem cell transplantation after CAR-T cell therapy. Data suggest that CAR-T cell therapy can be effectively used to bridge patient to transplant to improve long term outcome.
•• Lin JK, Lerman BJ, Barnes JI, Boursiquot BC, Tan YJ, Robinson AQL, et al. Cost-effectiveness of chimeric antigen receptor T cell therapy in relapsed or refractory pediatric B cell acute lymphoblastic leukemia. J Clin Oncol. 2018;36(32):3192–202 This is an important study that focuses on cost implications of CAR-T cell therapy. This study emphasizes the need for reducing cost of CAR-T cell therapy to make it universally accessible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Talha Badar declares that he has no conflict of interest.
Nirav N. Shah has received honoraria and/or travel support from Incyte, Celgene, and Miltenyi Biotec; has served on scientific advisory boards for Kite, Celgene, and Cellectar Biosciences; and has received institutional research support for clinical trials from Bristol-Myers Squibb and Miltenyi Biotec.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Leukemia
Rights and permissions
About this article
Cite this article
Badar, T., Shah, N.N. Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia. Curr. Treat. Options in Oncol. 21, 16 (2020). https://doi.org/10.1007/s11864-020-0706-6
Published:
DOI: https://doi.org/10.1007/s11864-020-0706-6