Opinion statement
Central nervous system gliomas are the most common primary brain tumor, and these are most often high-grade gliomas. Standard therapy includes a combination of surgery, radiation, and chemotherapy which provides a modest increase in survival, but virtually, no patients are cured, the overall prognosis remains poor, and new therapies are desperately needed. Tumor metabolism is a well-recognized but understudied therapeutic approach to treating cancers. Dietary and nondietary modulation of glucose homeostasis and the incorporation of dietary supplements and other natural substances are potentially important interventions to affect cancer cell growth, palliate symptoms, reduce treatment-associated side effects, and improve the quality and quantity of life in patients with cancer. These approaches are highly desired by patients. However, they can be financially burdensome, associated with toxicities, and have, on occasion, reduced the efficacy of proven therapies and negatively impacted patient outcomes. The lack of rigorous scientific data evaluating almost all diet and supplement-based therapies currently limits their incorporation into standard oncologic practice. Rigorous studies are needed to document and improve these potentially useful approaches in patients with brain and other malignancies.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS Statistical Report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14:v1–v49.
Stupp R, Mason W, van den Bent M, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. NEJM. 2005;352(10):987–96.
Tait MJ, Petrik V, Loosemore A, Bell BA, Papadopoulos MC. Survival of patients with glioblastoma multiforme has not improved between 1993 and 2004: analysis of 625 cases. Br J Neurosurg. 2007;21(5):496–500.
Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708.
Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–22.
Timbo BB, Ross MP, Mccarthy PV, Lin CTJ. Dietary supplements in a national survey: prevalence of use and reports of adverse events. J Am Diet Assoc. 2006;106(12):1966–74.
Tanvetyanon T, Bepler G. Beta-carotene in multivitamins and the possible risk of lung cancer among smokers versus former smokers: a meta-analysis and evaluation of national brands. Cancer. 2008;113(1):150–7.
Verhoef MJ, Hagen N, Pelletier G, Forsyth P. Alternative therapy use in neurologic diseases: use in brain tumor patients. Neurology. 1999;52(3):617–22.
Armstrong T, Cohen MZ, Hess KR, et al. Complementary and alternative medicine use and quality of life in patients with primary brain tumors. J Pain Symptom Manag. 2006;32(2):148–54.
Mani N. Electronic resources reviews: natural standard. J Med Libr Assoc. 2005;93(4):507–9.
Williams JT. Credible complementary and alternative medicine websites. J Adv Pr Oncol. 2013;4(2):123–4.
Ulbricht C, Weissner W, Hashmi S, et al. Essiac: systematic review by the natural standard research collaboration. J Soc Integr Oncol. 2009;7(2):73–80.
Ulbricht CE, Chao W. Phytochemicals in the oncology setting. Curr Treat Options Oncol. 2010;11(3-4):95–106.
Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men. JAMA. 2008;300(18):2123–33.
Klein EA, Thompson Jr IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer. JAMA. 2011;306(14):1549–56.
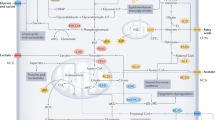
Seyfried TN, Flores R, Poff AM, D-Agostino DP. Cancer as a metabolic disease: implications for novel therapeutics. Carcinogenesis. 2013;35(3):515–27.
Warburg O. On the origins of cancer. Science. 1956;123(3191):309–14.
Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer. 2003;89(7):1375–82.
Oudard S, Arvelo F, Miccoli L, et al. High glycolysis in gliomas despite low hexokinase transcription and activity correlated to chromosome 10 loss. Br J Cancer. 1996;74(6):839–45.
Jelluma N, Yang X, Stokoe D, Evan GI, Dansen TB, Haas-Kogan DA. Glucose withdrawal induces oxidative stress followed by apoptosis in glioblastoma cells but not in normal human astrocytes. Mol Cancer Res. 2006;4(5):319–30.
Tisdale MJ, Brennan RA. Loss of acetoacetate coenzyme A transferase activity in tumours of peripheral tissues. Br J Cancer. 1983;47(2):293–7.
Fredericks M, Ramsey RB. 3-Oxo acid coenzyme A transferase activity in brain and tumors of the nervous system. J Neurochem. 1978;31(6):1529–31.
Maurer GD, Brucker DP, Bähr O, et al. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer. 2011;11(1):315.
Klement RJ, Kämmerer U. Is there a role for carbohydrate restriction in the treatment and prevention of cancer? Nutr Metab (Lond). 2011;8(1):75.
Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95.
Westhoff M-A, Karpel-Massler G, Brühl O, et al. A critical evaluation of PI3K inhibition in glioblastoma and neuroblastoma therapy. Mol Cell Ther. 2014;2:32.
Morfouace M, Lalier L, Oliver L, et al. Control of glioma cell death and differentiation by PKM2-Oct4 interaction. Cell Death Dis. 2014;5(1):e1036.
Neal EG, Chaffe H, Schwartz RH, et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500–6.
Henderson CB, Filloux FM, Alder SC, Lyon JL, Caplin DA. Efficacy of the ketogenic diet as a treatment option for epilepsy: meta-analysis. J Child Neurol. 2006;21(3):193–8.
Cervenka MC, Kossoff EH. Dietary treatment of intractable epilepsy. Continuum (Minneap Minn). 2013;19(3, Epilepsy):756–66.
Kossoff EH, Cervenka MC, Henry BJ, Haney CA, Turner Z. A decade of the modified Atkins diet (2003-2013): results, insights, and future directions. Epilepsy Behav. 2013;29(3):437–42.
Fine EJ, Segal-Isaacson CJ, Feinman RD, et al. Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients. Nutrition. 2012;28(10):1028–35.
Schmidt M, Pfetzer N, Schwab M, Strauss I, Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutr Metab (Lond). 2011;8(1):54.
Rieger J, Bähr O, Maurer GD, et al. ERGO: a pilot study of ketogenic diet in recurrent glioblastoma. Int J Oncol. 2014;45(6):1843–52. This study reports results of the one published study evaluating the safety and feasibility of a ketogenic diet in the management of recurrent glioblastoma.
Neal EG, Chaffe H, Schwartz RH, et al. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50(5):1109–17.
Gnagnarella P, Gandini S, La Vecchia C, Maisonneuve P. Glycemic index, glycemic load, and cancer risk: a meta-analysis. Am J Clin Nutr. 2008;87(6):1793–801.
Dong J-Y, Qin L-Q. Dietary glycemic index, glycemic load, and risk of breast cancer: meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011;126(2):287–94.
Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–82.
Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355(9206):773–8.
Williams LS, Rotich J, Qi R, et al. Effects of admission hyperglycemia on mortality and costs in acute ischemic stroke. Neurology. 2002;59(1):67–71.
Ali NA, O’Brien JM, Blum W, et al. Hyperglycemia in patients with acute myeloid leukemia is associated with increased hospital mortality. Cancer. 2007;110(1):96–102.
Villarreal-Garza C, Shaw-Dulin R, Lara-Medina F, et al. Impact of diabetes and hyperglycemia on survival in advanced breast cancer patients. Exp Diabetes Res. 2012;2012:Article ID 732027.
Sonabend RY, McKay SV, Okcu MF, Yan J, Haymond MW, Margolin JF. Hyperglycemia during induction therapy is associated with poorer survival in children with acute lymphocytic leukemia. J Pediatr. 2009;155(1):73–8.
Xu H, Zhang LM, Liu J, Ding GX, Ding Q, Jiang HW. The association between overall survival of prostate cancer patients and hypertension, hyperglycemia, and overweight in Southern China: a prospective cohort study. J Cancer Res Clin Oncol. 2013;139(6):943–51.
Hong YJ, Lim S, Jeon H, Lee S, Lee KH. Impact of hyperglycemia on survival and infection-related adverse events in patients with metastatic colorectal cancer who were receiving palliative chemotherapy. Cancer Res Treat. 2014;46(3):288–96.
Derr RL, Ye X, Islas MU, Desideri S, Saudek CD, Grossman SA. Association between hyperglycemia and survival in patients with newly diagnosed glioblastoma. J Clin Oncol. 2009;27(7):1082–6.
McGirt MJ, Chaichana KL, Gathinji M, et al. Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas. Neurosurgery. 2008;63(2):286–91.
Miranda-Goncalves V, Honavar M, Pinheiro C, et al. Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro Oncol. 2013;15(2):1923–35.
Colen CB, Shen Y, Ghoddoussi F, et al. Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo study. Neoplasia. 2011;13(7):620–32.
Seltzer MJ, Bennett BD, Joshi AD, et al. Inhibition of glutaminase preferentially slows growth of glioma cells with mutant IDH1. Cancer Res. 2010;70(22):8981–7.
Bittinger MA, Su SM, Fantin VR, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–30.
DeLorenze GN, McCoy L, Tsai A-L, et al. Daily intake of antioxidants in relation to survival among adult patients diagnosed with malignant glioma. BMC Cancer. 2010;10:215. doi:10.1186/1471-2407-10-215.
Kyritsis AP, Bondy ML, Levin VA. Modulation of glioma risk and progression by dietary nutrients and anti-inflammatory agents. Nutr Cancer. 2011;63(2):174–84.
Brem SS, Zagzag D, Tsanaclis AM, Gately S, Elkouby MP, Brien SE. Inhibition of angiogenesis and tumor growth in the brain. Suppression of endothelial cell turnover by penicillamine and the depletion of copper, an angiogenic cofactor. Am J Pathol. 1990;137(5):1121–42.
Sproull M, Brechbiel M, Camphausen K. Antiangiogenic therapy through copper chelation. Expert Opin Ther Targets. 2003;7(3):405–9. doi:10.1517/eott.7.3.405.22437.
Alpern-Elran H, Brem S. Angiogenesis in human brain tumors: inhibition by copper depletion. Surg Forum. 1985;36:498–500.
Brem S, Grossman SA, Carson KA, et al. Phase 2 trial of copper depletion and penicillamine as antiangiogenesis therapy of glioblastoma. Neuro Oncol. 2005;7(3):246–53.
Bouterfa H, Picht T, Kess D, et al. Retinoids inhibit human glioma cell proliferation and migration in primary cell cultures but not in established cell lines. Neurosurgery. 2000;46(2):419–30.
Dennis MK, Field AS, Burai R, et al. Phase II study of fenretinide (NSC 374551) in adults with recurrent malignant gliomas: a North American Brain Tumor Consortium Study. J Clin Oncol. 2004;22(21):4282–9.
Jaeckle KA, Hess KR, Yung WKA, et al. Phase II evaluation of temozolomide and 13-cis-retinoic acid for the treatment of recurrent and progressive malignant glioma: a North American Brain Tumor Consortium Study. J Clin Oncol. 2003;21(12):2305–11.
Penas-Prado M, Hess KR, Fisch MJ, et al. Randomized phase II adjuvant factorial study of dose-dense temozolomide alone and in combination with isotretinoin, celecoxib, and/or thalidomide for glioblastoma. Neuro Oncol. 2014;17(2):266–73. This study reports the results of a randomized phase II factorial study demonstrating no improvement in progression-free survival with the addition of several agents including isoretinoin and, in fact, showing that the addition of isoretinoin may be detrimental. Importantly, this study also demonstrates the utility and feasibility of the factorial design.
Salomón DG, Fermento ME, Gandini NA, et al. Vitamin D receptor expression is associated with improved overall survival in human glioblastoma multiforme. J Neurooncol. 2014;118(1):49–60.
Trouillas P, Honnorat J, Bret P, Jouvet A, Gerard JP. Redifferentiation therapy in brain tumors: long-lasting complete regression of glioblastomas and an anaplastic astrocytoma under long term 1-alpha-hydroxycholecalciferol. J Neurooncol. 2001;51(1):57–66.
Naidu KA, Liu Tang J, Akhilender Naidu K, Prockop LD, Nicosia SV, Coppola D. Antiproliferative and apoptotic effect of ascorbyl stearate in human glioblastoma multiforme cells: modulation of insulin-like growth factor-I receptor (IGF-IR) expression. J Neurooncol. 2001;54(1):15–22.
Tan PH, Sagoo P, Chan C, et al. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J Immunol. 2005;174(12):7633–44.
Purkayastha S, Berliner A, Fernando SS, et al. Curcumin blocks brain tumor formation. Brain Res. 2009;1266:130–8.
Weissenberger J, Priester M, Bernreuther C, et al. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res. 2010;16(23):5781–95.
Elamin MH, Shinwari Z, Hendrayani S-F, et al. Curcumin inhibits the Sonic Hedgehog signaling pathway and triggers apoptosis in medulloblastoma cells. Mol Carcinog. 2010;49(3):302–14.
Dhillon N, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491–9.
Winking M, Sarikaya S, Rahmanian A, Jödicke A, Böker DK. Boswellic acids inhibit glioma growth: a new treatment option? J Neurooncol. 2000;46(2):97–103.
Shen Y, Takahashi M, Byun HM, et al. Boswellic acid induces epigenetic alterations by modulating DNA methylation in colorectal cancer cells. Cancer Biol Ther. 2012;13(7):542–52.
Park B, Prasad S, Yadav V, Sung B, Aggarwal BB. Boswellic acid suppresses growth and metastasis of human pancreatic tumors in an orthotopic nude mouse model through modulation of multiple targets. PLoS One. 2011;6(10).
Cheng Y, Sk UH, Zhang Y, et al. Rational incorporation of selenium into temozolomide elicits superior antitumor activity associated with both apoptotic and autophagic cell death. PLoS One. 2012;7(4):1–10.
Wrobel JK, Seelbach MJ, Chen L, Power RF, Toborek M. Supplementation with selenium-enriched yeast attenuates brain metastatic growth. Nutr Cancer. 2013;65(4):563–70.
Pakdaman A. Symptomatic treatment of brain tumor patients with sodium selenite, oxygen, and other supportive measures. Biol Trace Elem Res. 1998;62(1-2):1–6.
Puspitasari IM, Abdulah R, Yamazaki C, Kameo S, Nakano T, Koyama H. Updates on clinical studies of selenium supplementation in radiotherapy. Radiat Oncol. 2014;9(1). This review summarizes data on 16 clinical studies conducted from 1987 to 2012 which evaluate the role of selenium supplementation in patients undergoing radiation therapy concluding that while there is suggestion of potential benefit in certain populations, data on the role of selenium in patients with brain tumors is not well defined.
Klein P, Tyrlikova I, Matthews GC. Dietary treatment in adults with refractory epilepsy: a review. Neurology. 2014;83(21):1978–85.
Compliance with Ethics Guidelines
Conflict of Interest
Roy E. Strowd III and Stuart A. Grossman declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Strowd, R.E., Grossman, S.A. The Role of Glucose Modulation and Dietary Supplementation in Patients With Central Nervous System Tumors. Curr. Treat. Options in Oncol. 16, 36 (2015). https://doi.org/10.1007/s11864-015-0356-2
Published:
DOI: https://doi.org/10.1007/s11864-015-0356-2