Abstract
Defining the appropriate sequencing of therapies for metastatic renal cell carcinoma (mRCC) has become increasingly complex in recent years given the approval of multiple targeted therapies. These targeted therapies fall into 2 broad mechanistic categories: (1) inhibitors of the mammalian target of rapamycin (mTOR), and (2) vascular endothelial growth factor (VEGF)-directed agents. In the current manuscript, data from relevant trials are reviewed to provide a context in which to use these agents across the first- and second-line setting. Strategies to incorporate promising agents currently in late stage development for mRCC are also described.
Opinion statement
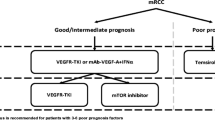
Currently, there is no consensus as to the optimal sequence of therapies for patients with metastatic renal cell carcinoma (mRCC). While interleukin-2 (IL-2) and temsirolimus are potential considerations for selected patients in the first-line setting, the majority of patients in this setting are likely candidates for vascular endothelial growth factor (VEGF)-directed therapies. Specifically, these therapies include sunitinib, pazopanib, and bevacizumab/interferon-α. Using the comparative data discussed herein, the relative merits of each should be discussed. In the second-line setting (following VEGF-directed therapy), axitinib, and everolimus are supported by phase III data. There is no data directly comparing the 2 agents—however, studies reviewed in the current manuscript (comparing VEGF- and mammalian target of rapamycin [mTOR]-directed approaches in the second-line setting) can potentially be used to inform clinical decision making.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–96.
Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–96.
Pal SK, Kortylewski M, Yu H, Figlin RA. Breaking through a plateau in renal cell carcinoma therapeutics: development and incorporation of biomarkers. Mol Cancer Ther. 2010;9:3115–25.
Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib vs interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. This study led to approval of sunitinib as first line therapy for clear cell renal cancer.
National Comprehensive Cancer Network Clinical Practice Guidelines: Renal Cell Carcinoma. Available at http://www.nccn.org. Accessed 29 Mar 2014. This provides the consensus guidelines of national and international experts for treatment of renal cancer.
Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer A, Escudier B. Randomized phase IIIB trial of temsirolimus and bevacizumab vs interferon and bevacizumab in metastatic renal cell carcinoma: results from INTORACT. Ann Oncol. 2012;23. [Abstract LBA21_PR].
Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28:2144–50. This trial led to approval of bevacizumab as a first-line option for kidney cancer.
Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. This study led to the approval of pazopanib as first line therapy for renal cell carcinoma.
Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. This trial led to the approval of everolimus as second line option of renal cancer progressed on VEGF TKi's.
Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib vs sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9. This trial led to the approval of axitinib as second-line option for renal cancer progressed on prior VEGF TKi's and also concluded that axitinib is better than sorafenib.
Motzer RJ, Hutson TE, Cella D, et al. Pazopanib vs sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31. Landmark trial showing no difference between 2 similar therapies as first-line agents for renal cancer though one better tolerated than other.
Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib vs sunitinib. N Engl J Med. 2014;370:1769–70. This is the comparative study which showed similar survival but different tolerability on 2 first-line agents.
Hutson TE, Escudier B, Esteban E, et al. Randomized Phase III trial of temsirolimus vs sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2013;32:760–7. This trial demonstrated that sequential VEGF therapies may portend longer overall survival.
Pal SK, Malangone E, Bhurke S, et al. Real-world treatment patterns in third-line patients with metastatic renal cell carcinoma (mRCC): the changing landscape in the United States. ASCO Meet Abstr. 2013;31:390.
Michel MS, Vervenne W, de Santis M, et al. SWITCH: a randomized sequential open-label study to evaluate efficacy and safety of sorafenib (SO)/sunitinib (SU) vs SU/SO in the treatment of metastatic renal cell cancer (mRCC). ASCO Meet Abstr. 2014;32:393.
Escudier B, Porta C, Bono P, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol. 2014;32:1412–8. Landmark crossover study which showed patient preference can help decide therapy between equally efficacious agents.
NCT01613846: phase III randomized sequential open-label study to evaluate the efficacy and safety of sorafenib followed by pazopanib versus pazopanib followed by sorafenib in the treatment of advanced / metastatic renal cell carcinoma. Available at http://www.clinicaltrials.gov. Accessed 12 Jul 2014.
NCT01684397: a phase I/ II trial of pazopanib alternating with bevacizumab in treatment-naive metastatic clear cell renal cell carcinoma patients. Available at http://www.clinicaltrials.gov.
Rini BI, Garcia JA, Cooney MM, et al. Toxicity of sunitinib plus bevacizumab in renal cell carcinoma. J Clin Oncol. 2010;28:e284–5.
NCT01668784: study of nivolumab (BMS-936558) vs. everolimus in pre-treated advanced or metastatic clear-cell renal cell carcinoma (CheckMate 025) Available at http://www.clinicaltrials.gov. Accessed 20 Feb 2014.
NCT01865747: a study of cabozantinib (XL184) vs everolimus in subjects with metastatic renal cell carcinoma (METEOR). Available at http://www.clinicaltrials.gov. Accessed 20 Feb 2014.
Pal SK, Hu A, Chang M, Figlin RA. Programmed death-1 inhibition in renal cell carcinoma: clinical insights and future directions. Clin Adv Hematol Oncol. 2014;12:90–9.
Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab for metastatic renal cell carcinoma (mRCC): results of a randomized, dose-ranging phase II trial. ASCO Meeting Abstracts. 2014;32.
Gibney GT, Aziz SA, Camp RL, et al. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol. 2013;24:343–9.
Albiges L, Guegan J, Le Formal A, et al. MET is a potential target across all papillary renal cell carcinomas. Result from a large molecular study of pRCC with CGHa and matching gene expression array. Clin Cancer Res. 2014;20:3411–21.
NCT01582672: phase 3 trial of autologous dendritic cell immunotherapy (AGS-003) plus standard treatment of advanced renal cell carcinoma (RCC) (ADAPT). Available at http://www.clinicaltrials.gov. Accessed 15 Dec 2013.
Amin A, Dudek A, Logan T, et al. Prolonged survival with personalized immunotherapy (AGS-003) in combination with sunitinib in unfavorable risk metastatic RCC (mRCC). ASCO Meet Abstr. 2013;31:357.
NCT01265901: a randomized, controlled phase III study investigating IMA901 multipeptide cancer vaccine in patients receiving sunitinib as first-line therapy for advanced/metastatic renal cell carcinoma. Available at http://clinicaltrials.gov/ct2/show/NCT01265901?term=IMA-901&rank=2. Accessed 22 Oct 2012.
Johnson T, Xu C-f, Choueiri TK, et al. Genome-wide association study (GWAS) of efficacy and safety endpoints in pazopanib- or sunitinib-treated patients with renal cell carcinoma (RCC). ASCO. Meet Abstr. 2014;32:4503.
Voss MH, Chen D, Marker M, et al. Identification and validation of predictive biomarkers (BM) for everolimus (EVE) in metastatic renal cell carcinoma: analysis of 442 patients on RECORD-3. ASCO Meet Abstr. 2014;32:4531.
Compliance with Ethics Guidelines
Conflict of Interest
Sumanta K. Pal received consulting fees from Novartis and Pfizer. Neeraj Agarwal and Parminder Singh declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Genitourinary Cancers
Rights and permissions
About this article
Cite this article
Singh, P., Agarwal, N. & Pal, S.K. Sequencing Systemic Therapies for Metastatic Kidney Cancer. Curr. Treat. Options in Oncol. 16, 3 (2015). https://doi.org/10.1007/s11864-014-0316-2
Published:
DOI: https://doi.org/10.1007/s11864-014-0316-2