Abstract
Aims
The term “fetal growth restriction (FGR)” is commonly used to describe fetuses with an estimated fetal weight that is less than 10th percentile for gestational age. This study aimed to investigate the longitudinal change of microRNA-590-3p (miR-590-3p), vascular endothelial growth factor (VEGF), placental growth factor (PIGF), and matrix metalloproteinase (MMP)9 expressions in early, middle, and late pregnancy, and their correlations with the fetal growth restriction (FGR) risk.
Methods
Totally, 970 pregnant women in early pregnancy were enrolled, and their plasma samples were, respectively, acquired in early pregnancy (at 10th or 11th week of gestational age), middle pregnancy (at 20th or 21st week of gestational age), and late pregnancy (at 33th or 34th week of gestational age) for miR-590-3p, VEGF, PIGF, and MMP9 determinations.
Results
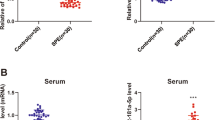
MiR-590-3p underwent a growing trend, but VEGF, PIGF, and MMP9 experienced declined trend along with pregnancy (all P < 0.001). Furthermore, the negative association of miR-590-3p with VEGF, PIGF, and MMP9 became stronger along with the pregnancy. Besides, miR-590-3p expression in middle and late pregnancy was higher, but VEGF, PIGF, and MMP9 expressions in middle and late pregnancy were lower in women affected by FGR compared to normal pregnant women (all P < 0.001). In addition, miR-590-3p, VEGF, PIGF, and MMP9 expression in middle and late pregnancy were of good value in predicting FGR risk.
Conclusions
miR-590-3p exhibits a growing trend during pregnancy, and its expression in middle and late pregnancy is associated with increased FGR risk via interaction with VEGF, PIGF, and MMP9.




Similar content being viewed by others

Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, Silver RM, Wynia K, Ganzevoort W (2016) Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet Gynecol 48(3):333–339. https://doi.org/10.1002/uog.15884
Albu AR, Anca AF, Horhoianu VV, Horhoianu IA (2014) Predictive factors for intrauterine growth restriction. J Med Life 7(2):165–171
Lindqvist PG, Molin J (2005) Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound Obstet Gynecol 25(3):258–264. https://doi.org/10.1002/uog.1806
Leite DFB, Cecatti JG (2019) Fetal growth restriction prediction: how to move beyond. Sci World J 2019:1519048. https://doi.org/10.1155/2019/1519048
Hu XQ, Zhang L (2019) MicroRNAs in uteroplacental vascular dysfunction. Cell 8(11):1344. https://doi.org/10.3390/cells8111344
Awamleh Z, Gloor GB, Han VKM (2019) Placental microRNAs in pregnancies with early onset intrauterine growth restriction and preeclampsia: potential impact on gene expression and pathophysiology. BMC Med Genomics 12(1):91. https://doi.org/10.1186/s12920-019-0548-x
Zhang Y, Pan X, Yu X, Li L, Qu H, Li S (2018) MicroRNA-590-3p inhibits trophoblast-dependent maternal spiral artery remodeling by repressing low-density lipoprotein receptor-related protein 6. Mol Genet Genomic Med 6(6):1124–1133. https://doi.org/10.1002/mgg3.491
Bao MH, Li GY, Huang XS, Tang L, Dong LP, Li JM (2018) Long noncoding RNA LINC00657 acting as a miR-590-3p sponge to facilitate low concentration oxidized low-density lipoprotein-induced angiogenesis. Mol Pharmacol 93(4):368–375. https://doi.org/10.1124/mol.117.110650
Mei B, Chen J, Yang N, Peng Y (2020) The regulatory mechanism and biological significance of the snail-miR590-VEGFR-NRP1 axis in the angiogenesis, growth and metastasis of gastric cancer. Cell Death Dis 11(4):241. https://doi.org/10.1038/s41419-020-2428-x
Zhu J, Zhong M, Pang Z, Yu Y (2014) Dysregulated expression of matrix metalloproteinases and their inhibitors may participate in the pathogenesis of pre-eclampsia and fetal growth restriction. Early Hum Dev 90(10):657–664. https://doi.org/10.1016/j.earlhumdev.2014.08.007
Ahmed A, Dunk C, Ahmad S, Khaliq A (2000) Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen–a review. Placenta 21:S16–S24. https://doi.org/10.1053/plac.1999.0524
Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marcal VM, Lobo TF, Peixoto AB, Araujo Junior E (2017) Fetal growth restriction: current knowledge. Arch Gynecol Obstet 295(5):1061–1077. https://doi.org/10.1007/s00404-017-4341-9
Gutierrez Garcia I, Perez Canadas P, Martinez Uriarte J, Garcia Izquierdo O, Angeles Jodar Perez M, de Guadiana G, Romualdo L (2018) D-dimer during pregnancy: establishing trimester-specific reference intervals. Scand J Clin Lab Invest 78(6):439–442. https://doi.org/10.1080/00365513.2018.1488177
Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and causes of preterm birth. Lancet 371(9606):75–84. https://doi.org/10.1016/S0140-6736(08)60074-4
Zhao X, Wei X, Wang X, Qi G (2020) Long noncoding RNA NORAD regulates angiogenesis of human umbilical vein endothelial cells via miR5903p under hypoxic conditions. Mol Med Rep 21(6):2560–2570. https://doi.org/10.3892/mmr.2020.11064
Li Y, Liu J (2020) MicroRNA-206 predicts raised fetal growth retardation risk through the interaction with vascular endothelial growth factor in pregnancies. Medicine (Baltimore) 99(7):e18897. https://doi.org/10.1097/MD.0000000000018897
Xu F, Xu S, Shao X, Wu M, Xu Y, Wang L (2017) Deficiency of calcium and microelements predict the risk of fetal growth restriction. Int J Clin Exp Med 10(5):7491–7499
American College of Obstetricians and Gynecologists (2019) ACOG Practice Bulletin No 204: Fetal Growth Restriction. Obstet Gynecol 133(2):e97–e109. https://doi.org/10.1097/AOG.0000000000003070
Barut F, Barut A, Gun BD, Kandemir NO, Harma MI, Harma M, Aktunc E, Ozdamar SO (2010) Intrauterine growth restriction and placental angiogenesis. Diagn Pathol 5:24. https://doi.org/10.1186/1746-1596-5-24
Regnault TR, de Vrijer B, Galan HL, Davidsen ML, Trembler KA, Battaglia FC, Wilkening RB, Anthony RV (2003) The relationship between transplacental O2 diffusion and placental expression of PlGF, VEGF and their receptors in a placental insufficiency model of fetal growth restriction. J Physiol 550(2):641–656. https://doi.org/10.1113/jphysiol.2003.039511
Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K, Ni W, Kalluri R, Werb Z (2013) Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci U S A 110(27):11109–11114. https://doi.org/10.1073/pnas.1309561110
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethic Committee of Shanghai First Maternity and Infant Hospital.
Consent to participate
All participants signed the informed consents before enrollment.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pei, J., Li, Y., Min, Z. et al. MiR-590-3p and its targets VEGF, PIGF, and MMP9 in early, middle, and late pregnancy: their longitudinal changes and correlations with risk of fetal growth restriction. Ir J Med Sci 191, 1251–1257 (2022). https://doi.org/10.1007/s11845-021-02664-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11845-021-02664-6