Abstract
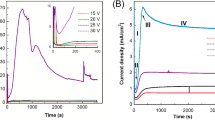
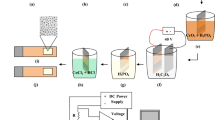
A hydrogel-based anodization system has been proposed, for the first time in the literature to our knowledge, to perform anodization. Here, the electrolyte solution is trapped inside the hydrogel, which makes the anodization process safer for the operators and environment. In addition, trapping the ions inside the hydrogel increases the reaction rate, and thus the process takes place in very short times. In the present study, the effects of the polymer concentration, process time, acid concentration and pressure were evaluated, and we found that the best porous structures with an average pore size of 26.7 nm were obtained for 4 M polymer concentration, 150 s process time, 410 kPa pressure and 10% (V/V) acid concentration. This study was preliminarily performed under hard anodization conditions, and in the future studies mild anodization conditions will be studied to be able to analyze the efficiency of this pioneering technique from all aspects.









Similar content being viewed by others
References
W. Lee, and S.J. Park, Chem. Rev. 114, 7487 (2014).
W. Lee, JOM 62, 57 (2010).
M.M. Lohrengel, Mater. Sci. Eng. R 11, 243 (1993).
H. Föll, M. Christophersen, J. Carstensen, and G. Hasse, Mater. Sci. Eng. R 39, 93 (2002).
J.W. Diggle, T.C. Downie, and C.W. Goulding, Chem. Rev. 69, 365 (1969).
S.R. Nicewarner-Peña, R.G. Freeman, B.D. Reiss, L. He, D.J. Peña, I.D. Walton, R. Cromer, C.D. Keating, and M.J. Natan, Science (80.-) 294, 137 (2001).
W. Lee, H. Han, A. Lotnyk, M.A. Schubert, S. Senz, M. Alexe, D. Hesse, S. Baik, and U. Gösele, Nat. Nanotechnol. 3, 402 (2008).
Z. Fan, H. Razavi, J.W. Do, A. Moriwaki, O. Ergen, Y.L. Chueh, P.W. Leu, J.C. Ho, T. Takahashi, L.A. Reichertz, S. Neale, K. Yu, M. Wu, J.W. Ager, and A. Javey, Nat. Mater. 8, 648 (2009).
D. Ding, Z. Chen, S. Rajaputra, and V. Singh, Sens. Actuators, B Chem. 124, 12 (2007).
Z. Huang, X. Zhang, M. Reiche, L. Ltu, W. Lee, T. Shimizu, S. Senz, and U. Gösele, Nano Lett. 8, 3046 (2008).
W. Lee, M. Alexe, K. Nielsch, and U. Gösele, Chem. Mater. 17, 3325 (2005).
H. Masuda, and K. Fukuda, Science (80-.) 268, 1466 (1995).
R.C. Furneaux, W.R. Rigby, and A.P. Davidson, Nature 337, 147 (1989).
F. Li, L. Zhang, and R.M. Metzger, Chem. Mater. 10, 2470 (1998).
Y. Lv, IOP Conf. Ser. Earth Environ. Sci. 100, 012022 (2017).
G.J. Strijkers, J.H.J. Dalderop, M.A.A. Broeksteeg, H.J.M. Swagten, and W.J.M. De Jonge, J. Appl. Phys. 86, 5141 (1999).
P. Skeldon, G.E. Thompson, S.J. Garcia-Vergara, L. Iglesias-Rubianes, and C.E. Blanco-Pinzon, Electrochem. Solid-State Lett. 9, B47 (2006).
S.J. Garcia-Vergara, P. Skeldon, G.E. Thompson, and H. Habazaki, Electrochim. Acta 52, 681 (2006).
E. Gonzalez, M. Paulis, M.J. Barandiaran, and J.L. Keddie, Langmuir 29, 2044 (2013).
O. Jessensky, F. Mü, and U. Gö, Appl. Phys. Lett. 72, 1173 (1998).
C.A. Schneider, W.S. Rasband, and K.W. Eliceiri, Nat. Methods 9, 671 (2012).
A. Gelir, E. Alveroglu, M. Tulun, Y. Yilmaz, and H. Sözeri, Polym. Eng. Sci. 50, 843 (2010).
S. Tabanli, A. Gelir, and Y. Yilmaz, Polym. Eng. Sci. 55, 406 (2015).
Y. Yilmaz, A. Gelir, F. Salehli, R.R. Nigmatullin, and A.A. Arbuzov, J. Chem. Phys. 125, 234705 (2006).
Y. Yilmaz, N. Uysal, A. Gelir, O. Guney, D.K. Aktas, S. Gogebakan, and A. Oner, Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 72, 332 (2009).
E. Alveroglu, A. Gelir, and Y. Yilmaz, Macromol. Symp. 281, 174 (2009).
Acknowledgement
This work was supported by the Scientific Research Projects of Istanbul Technical University (Grant No. TYL-2018-41075).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yilmaz, Y., Gelir, A., Gomleksiz, A. et al. Hydrogel Based Anodization: A Novel Technique to Form Ordered Nano-sized Porous Oxide Layer on the Aluminum Surface. JOM 74, 787–793 (2022). https://doi.org/10.1007/s11837-021-05081-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-05081-3