Abstract
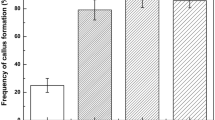
An improved protocol for high frequency plant regeneration via somatic embryogenesis from zygotic embryo-derived cell suspension cultures of watershield (Brasenia schreberi) was developed. Zygotic embryos formed pale-yellow globular structures and white friable callus at a frequency of 80% when cultured on half-strength MS medium supplemented with 0.3 mg l−1 2,4-D. However, the frequency of formation of pale-yellow globular structures and white friable callus decreased slightly with increasing concentrations of 2,4-D up to 3 mg l−1, where the frequency reached ~50% of the control. Cell suspension cultures from zygotic embryo-derived white friable callus were established using half-strength MS medium supplemented with 0.3 mg l−1 2,4-D. Upon plating of cell aggregates on half-strength MS basal medium, approximately 8.3% gave rise to somatic embryos and developed into plantlets. However, the frequency of plantlet development from cell aggregates was sharply increased (by up to 55%) when activated charcoal and zeatin were applied. Regenerated plantlets were successfully transplanted to potting soil and grown to normal plants in a growth chamber. The distinctive feature of this study is the establishment of a high frequency plant regeneration system via somatic embryogenesis from zygotic embryo-derived cell suspension cultures of watershield, which has not been previously reported. The protocol for plant regeneration of watershield through somatic embryogenesis could be useful for the mass propagation and transformation of selected elite lines.





Similar content being viewed by others
Abbreviations
- BA:
-
6-Benzyladenine
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- IAA:
-
Indole-3-acetic acid
- MS:
-
Murashige and Skoog
- NAA:
-
α-Naphthaleneacetic acid
References
Adams FS (1969) Winterbud production and fuction in Brasenia schreberi. Rhodora 71:417–433
Aliotta G, Monaco P, Pinto G, Pollio A, Previtera L (1991) Potential allelochemicals from Pistia stratiotes L. J Chem Ecol 17:2223–2234
Carlberg I, Glimelius K, Eriksson T (1983) Improved culture ability of potato protoplasts by use of activated charcoal. Plant Cell Rep 2:223–225
Cook CDK (1996) Aquatic plant book. SPB, Amsterdam
Dumas E, Monteuuis O (1995) In vitro rooting of micropropagated shoots from juvenile and mature Pinus pinaster explants: influence of activated charcoal. Plant Cell Tissue Organ Cult 40:231–235
Elakovich SD, Wooten JW (1987) An examination of the phytotoxicity of the water shield, Brasenia schreberi. J Chem Ecol 13:1935–1940
Endress PK (2005) Carpels of Brasenia (Cabombaceae) are completely ascidiate despite a long stigmatic crest. Ann Bot 96:209–215
Ervin GN, Wetzel RG (2003) An ecological perspective of allelochemical interference in land–water interface communities. Plant Soil 256:13–28
Fridborg G, Pedersen M, Landstrom LE, Eriksson T (1978) The effects of activated charcoal on tissue culture: absorption of metabolites inhibiting morphogenesis. Physiol Plant 43:104–106
Frodge JD, Thomas GL, Pauley GB (1990) Effects of canopy formation by floating and submergent aquatic macrophytes on the water quality of two shallow Pacific Northwest lakes. Aquat Bot 38:231–248
Ganesan M, Chandrasekar R, Ranjitha kumari BD, Jayabalan N (2007) Somatic embryogenesis and plant regeneration of Abelmoschus esculentus through suspension culture. Biol Plant 51:414–420
Guo Y, Bai J, Zhang Z (2007) Plant regeneration from embryogenic suspension-derived protoplasts of ginger (Zingiber officinale Rosc.). Plant Cell Tissue Organ Cult 89:151–157
Gürel S, Gürel E, Kaya Z (2000) Doubled haploid plant production from unpollinated ovules of sugar beet (Beta vulgaris L.). Plant Cell Rep 19:1155–1159
Hamana K, Niitsu M, Samejima K (1998) Unusual polyamines in aquatic plants: the occurrence of homospermidine, norspermidine, thermospermine, norspermine, aminopropylhomospermidine, bis(aminopropyl)ethanediamine, and methylspermidine. Can J Bot 76:130–133
Kakuta M, Misaki A (1979) A polysaccharide of ‘Junsai’ (Brasenia schreberi J. F. Gmel) mucilage: constitution and linkage analysis. Agric Biol Chem 43:993–1005
Kamo K, Jones B, Castillon J, Bolar J, Smith F (2004) Dispersal and size fractionation of embryogenic callus increases the frequency of embryo maturation and conversion in hybrid tea roses. Plant Cell Rep 22:787–792
Kelly MS, Pinder JE III (1996) Foliar uptake of 137Cs from the water column by aquatic macrophytes. J Environ Radioact 30:271–280
Khanna P (1965) Morphological and embryological studies in Nymphaeaceae II. Brasenia schreberi Gmel. and Nelumbo nucifera Gaertn. Aust J Bot 13:379–387
Kunitake H, Nakashima T, Mori K, Tanaka M, Mii M (1995) Plant regeneration from mesophyll protoplasts of Lisianthus (Eusroma gradiflorum) by adding activated charcoal into protoplasts culture medium. Plant Cell Tissue Organ Cult 43:59–65
Lee J-S, Kim C, Kim IS, Lee EJ, Choi H-K (2006) In vitro regeneratioin of Phragmites australis through embryogenic cultures. J Plant Biotechnol 8:21–25
Lu Z, Zhu J, Zhu S, Chen Z (1984) Preliminary studies on the beetle (Galerucella birmanica Jacoby)—an insect pest of water chestnut and water shield (in Chinese). Sci Agric Sin 5:73–76
Martini KP, Shahanazbeegum A, Zhang CL, Slater A, Madhusoodanan PV (2007) In vitro propagation of Ophiorrhiza prostrata through somatic embryogenesis. Biol Plant 51:769–772
Misaki A, Smith F (1962) Structure of the polysaccharide of the Japanese water plant, Brasenia schreberi J. F. Gmel. J Agric Food Chem 10:104–108
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nhut DT, Le BV, Fukai S, Tanaka M, Tran Thanh Van K (2001) Effects of activated charcoal, explant size, explant position and sucrose concentration on plant and shoot regeneration of Lilium longiflorum via young stem culture. Plant Growth Regul 33:59–65
Osborn JM, Schneider EL (1988) Morphological studies of the Nymphaeaceae sensu lato XVI. The floral biology of Brasenia schreberi. Ann Mo Bot Gard 75:778–794
Park CW (2005) The genera of vascular plants of Korea. Academy, Seoul
Pinder JE III, Hinton TG, Whicker FW (2006) Foliar uptake of cesium from the water column by aquatic macrophytes. J Environ Radioact 85:23–47
Rout GR, Samantaray S, Das P (1995) Somatic embryogenesis and plant regeneration from callus culture of Acacia catechu: a multipurpose leguminous tree. Plant Cell Tissue Organ Cult 42:283–285
Rueb S, Leneman M, Schilperoort RA, Hensgens LAM (1994) Efficient plant regeneration through somatic embryogenesis from callus induced on mature rice embryo (Oryza sativa L.). Plant Cell Tissue Organ Cult 36:259–264
Samantaray S, Rout GR, Das P (1997) Regeneration of plants via somatic embryogenesis from leaf base and leaf tip segments of Echinochloa colona. Plant Cell Tissue Organ Cult 47:119–125
Staikidou I, Selby C, Hanks G (2006) Stimulation of in vitro bulblet growth in Galanthus species with sucrose and activated charcoal. Acta Hortic 725:421–426
Taylor ML, Osborn JM (2006) Pollen ontogeny in Brasenia (Cabombaceae, Nymphaeales). Am J Bot 93:344–356
Teng WL (1997) Activated charcoal affects morphogenesis and enhances sporophyte regeneration during leaf cell suspension culture of Platycerium bifurcatum. Plant Cell Rep 17:77–83
Teixeria JB, Sondahl MR, Kirby EG (1994) Somatic embryogenesis from immature inflorescences of oil palm. Plant Cell Rep 13:247–250
Weatherhead MA, Burdon J, Henshaw GG (1979) Effects of activated charcoal as an additive plant tissue culture media. Z Pflanzenphysiol 94:399–405
Acknowledgments
This work was supported by a grant from KRIBB Research Initiative Program, and a grant from the Korea Science and Engineering Foundation (BDW0800723) and the Korea Institute of Environment Science and Technology (EGW0600712).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oh, M.J., Na, H.R., Choi, HK. et al. High frequency plant regeneration from zygotic-embryo-derived embryogenic cell suspension cultures of watershield (Brasenia schreberi). Plant Biotechnol Rep 2, 87–92 (2008). https://doi.org/10.1007/s11816-008-0047-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-008-0047-6