Abstract
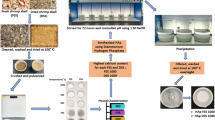
Oyster shells are abundantly available in nature without eminent use and are dumped into landfills in vast quantities. Their improper disposal causes environmental problems, resulting in a waste of natural resources. Recycling shell waste could potentially eliminate the environmental problems and, moreover, convert the waste into high-valueadded products, such as synthetic precipitated calcium carbonate (PCC), which can be obtained from oyster waste and which is used to enhance the mechanical properties of various materials. It can also be used as a filler material in the plastic and paper industries. This study presents a simple method for the extraction of aragonite needles from oyster shell waste via a carbonation process. The obtained aragonite-precipitated calcium carbonate (PCC) is characterized by XRD and SEM, which is used to assess the morphology and particle size. Using the proposed process, oyster shell waste powder was calcined at 1,000 °C for 2 h, after which the calcined shell powder was dissolved in water for hydration. The hydrated solution was mixed with an aqueous solution of magnesium chloride at 80 °C and CO2 was then bubbled into the suspension for 3 h to produce needle-shaped aragonite PCC. Finally, aragonite-type precipitated calcium carbonate was synthesized from the oyster shell powder via a simple carbonation process, yielding a product with an average particle size of 30-40 μm.
Similar content being viewed by others
References
P. Bernal and D. Oliva, The first global integrated marine assessment. World Ocean assessment 1. Chapter 12, United Nations, New York (2016).
FAO, The state of world fisheries and aquaculture, Food and Agriculture Organization of the United Nations (2014).
FAO, Global Aquaculture Production statistics database updated to 2013 Summary information, Food and Agriculture Organization of the United Nations (2015).
H. S. Kim, The study of application of discarded oyster shell powder as an architectural material, Master thesis, Korea (2007).
G. L. Yoon, B.T. Kim, B.O. Kim and S. H. Han, Waste Manage., 23, 825 (2003).
J. H. Jung, K. S. Yoo, H. G. Kim, H. K. Lee and B. H. Shon, J. Ind. Eng. Chem., 13, 512 (2007).
H. B. Kwon, C.W. Lee, B. S. Jun, J.D. Yun, S.Y. Weon and B. Koopman, Resour. Conserv. Recy., 41, 75 (2004).
S. Asaoka, T. Yamamoto, S. Kondo and S. Hayakawa, Bioresour. Technol., 100, 4127 (2009).
W. H. Park and C. Polprasert, Ecol. Eng., 34, 50 (2008).
N. Nakatani, H. Takamori, K. Takeda and H. Sakugawa, Bioresour. Technol., 100, 1510 (2009).
C.H. Lee, D.K. Lee, M.A. Ali and P. J. Kim, Waste Manage., 28, 2702 (2008).
H. B. Kwon, C.W. Lee, B. S. Jun, S.Y. Weon and B. Koopman, Resour. Conserv. Recy., 41, 75 (2004).
K. Mori and K. Takahashi, Mizushorigijutu., 39, 1 (1998).
R.A. F. de Alvarenga, B.M. Galindro Cde. F. Helpa and S.R. Soares, J. Environ. Manage., 106, 102 (2012).
J. Hutchinson and A.D. O’Sullivan, Scanning electron microscopy of substrates from bioengineered treatment reactors, University of Canterbury, New Zealand (2008).
L. Xiang, Y. Xiang, Y. Wen and F. Wei, Mater. Lett., 58, 959 (2004).
Z.T. Yao, T. Chen, H.Y. Li, M. S. Xia, Y. Ye and H. Zheng, J. Hazard. Mater., 262, 212 (2013).
M. Suzuki, S. Sakuda and H. Nagasawa, Biosci. Biotech. Bioch., 71, 1735 (2007).
Z.T. Yao, M. S. Xia, H.Y. Li, T. Chen, Y. Ye and H. Zheng, Crit. Rev. Env. Sci. Tec., 44, 2502 (2014).
I. Corni, T. J. Harvey, J. A. Wharton, K.R. Stokes, F. C. Walsh and R. J.K. Wood, Bioinspir. Biomim., 7, 1 (2012).
M.H. Chong, B.C. Chun, Y.C. Chung and B.G. Cho, J. Appl. Polym. Sci., 99, 1583 (2006).
Z.D. Lin, Z. X. Guan, C. Chen and B. F. Xu, Thermochim. Acta., 551, 149 (2013).
E. Daniel and P. Luiz Antonio, Mater. Res. Bull., 12, 517 (2009).
H.Y. Li, Y.Q. Tan, L. Zhang, Y. X. Zhang, Y. H. Song, Y. Ye and M. S. Xia, J. Hazard. Mater., 217, 256 (2012).
P.M.A. de Melo, L.B. Silva, A.S.F. Santos, T.A. Passos, S. J.G. Lima and M. M. Ueki, Proceedings of 22nd International Congress of Mechanical Engineering, Ribeirao Preto (2013).
J.W. Ahn, W.K. Park, K.S. You, H.C. Cho, S. J. Ko and C. Han, Solid State Phenom., 124, 707 (2007).
W.K. Park, S. J. Ko, S.W. Lee, K. H. Cho, J.W. Ahn and C. Han, J. Cryst. Growth, 310, 2593 (2008).
M. Holcomb, A. L. Cohen, R. I. Gabitov and J. L. Hutter, Geochim. Cosmochim. Act., 73, 4166 (2009).
C. Linga Raju, K.V. Narasimhulu, N.O. Gopal, J. L. Rao and B.C.V. Reddy, J. Mole. Structure., 608, 201 (2002).
J. Kuther, G. Nelles, R. Seshadri, M. Schaub, H. J. Butt and W. Tremel, Chem. Eur. J., 4, 1834 (1998).
Y. Ota, S. Inui, T. Iwashita, T. Kasuga and Y. Abe, J. Am. Ceram. Soc., 78, 1983 (1995).
L. F. Wang, I. Sondi and E. Matijevic, J. Colloid Interface Sci., 218, 545 (1999).
S.D. Skapin and I. Sondi, J. Colloi Interface Sci., 347, 221 (2010).
Z. S. Hu and Y.L. Deng, J. Colloid Interface Sci., 266, 359 (2003).
D. Rautaray, A. Banpurkar, S.R. Sainkar, A.V. Limaye, N.R. Pavaskar, S.B. Ogale and M. Sastry, Adv. Mater., 15, 1273 (2003).
G. Li, Z. Li and H.W. Ma, Int. J. Min. Proc., 123, 25 (2013).
C. Domingo, E. Loste, J. Gomez-Morales, J. Garcia-Carmona and J. Fraile, J. Super, Flu., 36, 202 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramakrishna, C., Thenepalli, T., Han, C. et al. Synthesis of aragonite-precipitated calcium carbonate from oyster shell waste via a carbonation process and its applications. Korean J. Chem. Eng. 34, 225–230 (2017). https://doi.org/10.1007/s11814-016-0264-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-016-0264-6