Abstract
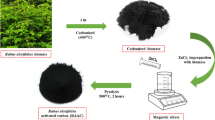
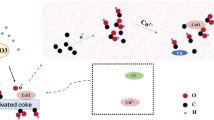
The preparation of activated carbon sorbent for Hg removal was simplified by combining activation and functionalization processes into one step. Jujube-based carbon material was first mixed with CuCl2 solution and then activated for the preparation of Cu-impregnated activated carbon. Physical and chemical properties of prepared activated carbon were investigated by means of N2 adsorption, SEM-EDS, XRD. A fixed-bed reactor with CEMS (Continuous emission monitoring system) was used to test the Hg adsorption ability of prepared activated carbon. DFT (Density functional theory) method of computational chemistry calculation was applied to identify the Hg adsorption mechanisms on sorbent surface.
Similar content being viewed by others
References
M. C. Houston, J. Clin. Hypertens., 13, 621 (2011).
United Nations Environment Programme, The Global Hg Assessment 2013, Switzerland (2013).
Q. Zhou, Y. Duan, C. Zhu, J. Zhang, M. She, H. Wei and Y. Hong, Korean J. Chem. Eng., 32, 1405 (2015).
Natural Resources Defense Council, Summary of Recent Mercury Emission Limits for Power Plants in the United States and China, Unites States and China (2012).
A. P. Jones, J. W. Hoffmann, D. N. Smith, T. J. Feeley and J. T. Murphy, Environ. Sci. Technol., 41, 1365 (2007).
H. Yang, Z. Xu, M. Fan, A. E. Bland and R. R. Judkins, J. Hazard. Mater., 146, 1 (2007).
H. Hadoun, Z. Sadaoui, N. Souami, D. Sahel and I. Toumert, Appl. Surf. Sci., 280, 1 (2013).
S. A. Dastgheib, J. Ren, M. Rostam-Abadi and R. Chang, Appl. Surf. Sci., 290, 92 (2014).
C. Chiu et al., Aerosol Air Qual., Res., 2094, 15 (2015).
L. Zhang, Y. Zhuo, W. Du, Y. Tao, C. Chen and X. Xu, Ind. Eng. Chem. Res., 51, 5292 (2012).
J. F. González, S. Román, C. M. González-García, J. M. V. Nabais and A. L. Ortiz, Ind. Eng. Chem. Res., 48, 7474 (2009).
J. D. Laumb, S. A. Benson and E. A. Olson, Fuel Process. Technol., 85, 577 (2004).
R. Landreth, S. Nelson, X. Liu, Z. Tang, A. Overholt and L. Brickett, World of Coal Ash (2007).
F. Goodarzi, J. Environ. Monit., 6, 792 (2004).
J. Wang, F. Xue, Y. Liu, Physical chemistry, Tsinghua University Press, Beijing (1992).
L. Tao, X. Guo and C. Zheng, P. Combust. Inst., 34, 2803 (2013).
J. A. Steckel, Phys. Rev. B., 77, 115412 (2008).
L. Geng, L. Han, W. Cen, J. Wang, L. Chang, D. Kong and G. Feng, Appl. Surf. Sci., 321, 30 (2014).
M. D. Segall, P. Lindan, M. J. Probert, C. J. Pickard, P. J. Hasnip, S. J. Clark and M. C. Payne, J. Phys.-Condens. Mat., 14, 2717 (2002).
S. J. Clark, M. D. Segall, C. J. Pickard, P. J. Hasnip, M. J. Probert, K. Refson and M. C. Payne, Z. Kristallogr., 220, 567 (2005).
J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 77, 3865 (1996).
H. J. Monkhorst and J. D. Pack, Phys. Rev. B., 13, 5188 (1976).
S. Ramos De Debiaggi, G. F. Cabeza, C. D. Toro, A. M. Monti, S. Sommadossi and A. F. Guillermet, J. Alloy. Compd., 509, 3238 (2011).
K. Lejaeghere, V. Van Speybroeck, G. Van Oost and S. Cottenier, Crit. Rev. Solid State, 39, 1 (2014).
A. Soon, M. Todorova, B. Delley and C. Stampfl, Phys. Rev. B., 73 (2006).
B. Zhang, J. Liu, C. Zheng and M. Chang, Chem. Eng. J., 256, 93 (2014).
A. E. Reed, F. Weinhold, L. A. Curtiss and D. J. Pochatko, J. Chem. Phys., 84, 5687 (1986).
W. Xiang, J. Liu, M. Chang and C. Zheng, Chem. Eng. J., 200-202, 91 (2012).
B. Zhang, J. Liu, C. Zheng and M. Chang, Chem. Eng. J., 256, 93 (2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, Y., Zhuo, Y., Zhu, Z. et al. Density functional theory study on Hg removal mechanisms of Cu-impregnated activated carbon prepared by simplified method. Korean J. Chem. Eng. 33, 2869–2877 (2016). https://doi.org/10.1007/s11814-016-0153-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-016-0153-z