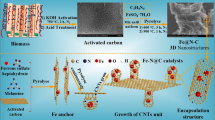
Abstract
Iron-based metal organic frameworks have been verified to be efficient heterogeneous Fenton catalysts due to their open pore channels and highly uniform distribution of metallic centers. In these catalysts, however, the iron element is mainly in the form of Fe(III), which results in a process required to reduce Fe(III) to Fe(II) to initiate Fenton reaction. To address this problem, carbon nanotubes (CNTs) with electron-rich oxygen-functional groups on the surface were incorporated into the metal organic frameworks (MIL-88B-Fe) to improve Fe(II) content for an enhanced Fenton-like performance. The prepared CNT@MIL-88B-Fe (C@M) showed much stronger catalytic ability toward H2O2 than MIL-88B-Fe. The pseudo-first-order kinetic constant for phenol degradation by C@M (0.32 min–1) was about 7 times that of MIL-88B-Fe, and even higher than or comparable to the values of reported heterogeneous Fenton-like catalysts. Moreover, the Fenton-like system could effectively degrade various kinds of refractory organic pollutants and exhibited excellent catalytic activity over a wide pH range (4–9). XPS analysis confirmed that Fe(II) content of the catalyst gradually increased with CNT loadings. Electron spin resonance analysis showed that the signal intensity (•OH) of C@M was much higher than MIL-88B-Fe, which was consistent with the degradation efficiency of pollutants. Furthermore, the Fe(II) content of the catalyst gradually increased along with the oxygen-functional group content of CNTs. The result demonstrated that oxygen-containing functional groups of CNTs have a significant impact on the enhanced catalytic performance of C@M. This study provides a new insight to enhance Fenton reaction by using nanocarbon materials.

Similar content being viewed by others
References
Araya T, Jia M, Yang J, Zhao P, Cai K, Ma W H, Huang Y P (2017). Resin modified MIL-53 (Fe) MOF for improvement of photocatalytic performance. Applied Catalysis B: Environmental, 203: 768–777
Branca C, Frusteri F, Magazù V, Mangione A (2004). Characterization of carbon nanotubes by TEM and Infrared Spectroscopy. The Journal of Physical Chemistry B, 108(11): 3469–3473
Chen D Z, Chen S S, Jiang Y J, Xie S S, Quan H Y, Hua L, Luo X B, Guo L (2017). Heterogeneous Fenton-like catalysis of Fe-MOF derived magnetic carbon nanocomposites for degradation of 4-nitrophenol. RSC Advances, 7(77): 49024–49030
Duan H T, Liu Y, Yin X H, Bai J F, Qi J (2016). Degradation of nitrobenzene by Fenton-like reaction in a H2O2/schwertmannite system. Chemical Engineering Journal, 283: 873–879
Duarte F, Maldonado-Hódar F J, Pérez-Cadenas A F, Madeira L M (2009). Fenton-like degradation of azo-dye orange II catalyzed by transition metals on carbon aerogels. Applied Catalysis B: Environmental, 85(3): 139–147
Gao C, Chen S, Quan X, Yu H T, Zhang Y B (2017). Enhanced Fentonlike catalysis by iron-based metal organic frameworks for degradation of organic pollutants. Journal of Catalysis, 356: 125–132
Gonzalez-Olmos R, Holzer F, Kopinke F D, Georgi A (2011). Indications of the reactive species in a heterogeneous Fenton-like reaction using Fe-containing zeolites. Applied Catalysis A: General, 398(1): 44–53
Hou X, Huang X, Ai Z, Zhao J, Zhang L (2016). Ascorbic acid/Fe@Fe2O3: A highly efficient combined Fenton reagent to remove organic contaminants. Journal of Hazardous Materials, 310: 170–178
Ji Y, Huang L, Hu J, Streb C, Song Y F (2015). Polyoxometalatefunctionalized nanocarbon materials for energy conversion, energy storage and sensor systems. Energy & Environmental Science, 8(3): 776–789
Jia Z, Duan X G, Qin P, Zhang W C, Wang W M, Yang C, Sun H Q, Wang S B, Zhang L C (2017a). Sordered atomic packing structure of metallic glass: Toward ultrafast hydroxyl radicals production rate and strong electron transfer ability in catalytic performance. Advanced Functional Materials, 27(38): 1–9
Jia Z, Wang J C, Liang S X, Zhang WC, Wang WM, Zhang L C (2017b). Activation of peroxymonosulfate by Fe78Si9B13 metallic glass: The influence of crystallization. Journal of Alloys and Compounds, 728: 525–533
Jin H, Tian X, Nie Y, Zhou Z, Yang C, Li Y, Lu L (2017). Oxygen vacancy promoted heterogeneous Fenton-like degradation of ofloxacin at pH 3.2–9.0 by Cu substituted magnetic Fe3O4@FeOOH nanocomposite. Environmental Science & Technology, 51(21): 12699–12706
Kim J, Kim S N, Jang H G, Seo G, Ahn WS (2013). CO2 cycloaddition of styrene oxide over MOF catalysts. Applied Catalysis A: General, 453: 175–180
Kim K, Zhu P, Li N, Ma X, Chen Y (2011). Characterization of oxygen containing functional groups on carbon materials with oxygen Kedge X-ray absorption near edge structure spectroscopy. Carbon, 49 (5): 1745–1751
Laurier K G M, Vermoortele F, Ameloot R., DeVos D E, Hofkens J, Roeffaers M B J (2013). Iron(III)-based metal–organic frameworks as visible light photocatalysts. Journal of the American Chemical Society, 135(39): 14488–14491
Li Y H, Xu C, Wei B, Zhang X, Zheng M, Wu D, Ajayan P M (2002). Self-organized ribbons of aligned carbon nanotubes. Chemistry of Materials, 14(2): 483–485
Liang S X, Jia Z, Liu Y J, Zhang W C, Wang W M, Lu J, Zhang L C (2018a). Compelling rejuvenated catalytic performance in metallic glasses. Advanced Materials, 1802764: 1–11
Liang S X, Jia Z, Zhang W C, Li X F, Wang W M, Lin H C, Zhang L C (2018b). Ultrafast activation efficiency of three peroxides by Fe78SiB13 metallic glass under photo-enhanced catalytic oxidation: A comparative study. Applied Catalysis B: Environmental, 221: 108–118
Lu Y B, He S L, Wang D T, Luo S Y, Liu A P, Luo H P, Liu G L, Zhang R D (2018). A pulsed switching peroxi-coagulation process to control hydroxyl radical production and enhance 2,4-Dichlorphenoxyacetic acid degradation. Frontiers of Environmental Science & Engineering, 12(5): 9
Lv H, Zhao H, Cao T, Qian L, Wang Y, Zhao G (2015). Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. Journal of Molecular Catalysis A: Chemical, 400: 81–89
Lyu L, Zhang L, Hu C, Yang M (2016). Enhanced Fenton-catalytic efficiency by highly accessible active sites on dandelion-like copperaluminum-silica nanospheres for water purification. Journal of Materials Chemistry A, 4(22): 8610–8619
Ma M, Noei H, Mienert B, Niesel J, Bill E, Muhler M, Fisher R A, Wang Y M, Schatzschneider U, Metzler-Nolte N (2013). Iron metal–organic frameworks MIL-88B and NH2-MIL-88B for the loading and delivery of the gasotransmitter carbon monoxide. Chemistry–A European Journal, 19(21): 6785–6790
Mao J, Quan X, Wang J, Gao C, Chen S, Yu H T, Zhao Y B (2018). Enhanced heterogeneous Fenton-like activity by Cu-doped BiFeO3 perovskite for degradation of organic pollutants. Frontiers of Environmental Science & Engineering, 12(6): 10
Navalon S, Martin R, Alvaro M, Garcia H (2010). Gold on diamond nanoparticles as a highly efficient Fenton catalyst. Angewande Chemie International Edition, 49(45): 8403–8407
Ou X X, Yan J F, Zhang F J, Zhang C H (2018). Accelerated degradation of orange G over a wide pH range in the presence of FeVO4. Frontiers of Environmental Science & Engineering, 12(1): 7
Peng J, Xue J, Li J,Du Z, Wang Z, Gao S (2017). Catalytic effect of low concentration carboxylated multi-walled carbon nanotubes on the oxidation of disinfectants with Cl-substituted structure by a Fentonlike system. Chemical Engineering Journal, 321: 325–334
Qin Y, Zhang L, An T (2017). Hydrothermal carbon-mediated Fentonlike reaction mechanism in the degradation of alachlor: direct electron transfer from hydrothermal carbon to Fe(III). Applied Materials & Interfaces, 9: 17115–17124
Restivo J, Órfão J J M, Armenise S, Garcia-Bordejé E, Pereira M F R (2012). Catalytic ozonation of metolachlor under continuous operation using nanocarbon materials grown on a ceramic monolith. Journal of Hazardous Materials, 239–240: 249–256
Shearer C J, Alexey C, Dominik E (2014). Application and future challenges of functional nanocarbon hybrids. Advanced Materials, 26(15): 2295–2318
Sun J H, Sun S P, Fan M H, Guo H Q, Qiao L P, Sun R X (2007). A kinetic study on the degradation of p-nitroaniline by Fenton oxidation process. Journal of Hazardous Materials, 148(1): 172–177
Sun M, Chu C, Geng F, Lu X, Qu J, Crittenden J, Elimelech M, Kim J H (2018). Reinventing Fenton chemistry: Iron oxychloride nanosheet for pH-insensitive H2O2 activation. Environmental Science & Technology Letters, 5(3): 186–191
Tang J, Wang J (2018). Metal organic framework with coordinatively unsaturated sites as efficient Fenton-like catalyst for enhanced degradation of sulfamethazine. Environmental Science & Technology, 52(9): 5367–5377
Tian S, Zhang J, Chen J, Kong L, Lu J, Ding F, Xiong Y (2013). Fe2(MoO4)3 as an effective photo-Fenton-like catalyst for the degradation of anionic and cationic dyes in a wide pH range. Industrial & Engineering Chemistry Research, 52(37): 13333–13341
Wang D W, Su D (2014). Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy & Environmental Science, 7(2): 576–591
Wang J, Bai Z (2017). Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chemical Engineering Journal, 312: 79–98
Wang X, Wang G, Chen S, Fan X, Quan X, Yu H (2017). Integration of membrane filtration and photoelectrocatalysis on g-C3N4/CNTs/Al2O3 membrane with visible-light response for enhanced water treatment. Journal of Membrane Science, 541: 153–161
Wang Y, Zhao H, Zhao G (2015). Iron-copper bimetallic nanoparticles embedded within ordered mesoporous carbon as effective and stable heterogeneous Fenton catalyst for the degradation of organic contaminants. Applied Catalysis B: Environmental, 164: 396–406
Wu Y Y, Yang C X, Yan X P (2014). Fabrication of metal-organic framework MIL-88B films on stainless steel fibers for solid-phase microextraction of polychlorinated biphenyls. Journal of Chromatography A, 1334: 1–8
Yang D Q, Rochette J F, Sacher E (2005). Functionalization of multiwalled carbon nanotubes by mild aqueous sonication. The Journal of Physical Chemistry B, 109(16): 7788–7794
Yang Z, Yu A, Shan C, Gao G, Pan B (2018). Enhanced Fe(III)-mediated Fenton oxidation of atrazine in the presence of functionalized multiwalled carbon nanotubes. Water Research, 137(15): 37–46
Yin D, Zhang L, Zhao X, Chen H, Zhai Q (2015). Iron-glutamatesilicotungstate ternary complex as highly active heterogeneous Fenton-like catalyst for 4-chlorophenol degradation. Chinese Journal of Catalysis, 36(12): 2203–2210
Yu L, Yang X, Ye Y, Wang D (2015). Efficient removal of atrazine in water with a Fe3O4/MWCNTs nanocomposite as a heterogeneous Fenton-like catalyst. RSC Advances, 5(57): 46059–46066
Zhang G, Gao Y, Zhang Y, Guo Y (2010). Fe2O3-pillared rectorite as an efficient and stable Fenton-Like heterogeneous catalyst for photodegradation of organic contaminants. Environmental Science & Technology, 44(16): 6384–6389
Zhang L, Nie Y, Hu C, Qu J (2012). Enhanced Fenton degradation of Rhodamine B over nanoscaled Cu-doped LaTiO3 perovskite. Applied Catalysis B: Environmental, 125(21): 418–424
Zhang X Y, Ding Y B, Tang H Q, Han X Y, Zhu L H, Wang N (2014). Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: Efficiency, stability and mechanism. Chemical Engineering Journal, 236(15): 251–262
Zhou H, Shen Y F, Wang J Y, Chen X, O’Young C L, Suib S L (1998). Studies of decomposition of H2O2 over manganese oxide octahedral molecular sieve materials. Journal of Catalysis, 176(2): 321–328
Zhou R, Shen N, Zhao J, Su Y, Ren H (2018). Glutathione-coated Fe3O4 nanoparticles with enhanced Fenton-like activity at neutral pH for degrading 2,4-dichlorophenol. Journal of Materials Chemistry A, 6 (3): 1275–1283
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 51478075), Department of Science & Technology of Dalian (No. 2018J11CY012), the Program of Introducing Talents of Discipline to Universities (No. B13012), and programme for Changjiang Scholars and Innovative Research Team in University (No. IRT_13R05).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhang, H., Chen, S., Zhang, H. et al. Carbon nanotubes-incorporated MIL-88B-Fe as highly efficient Fenton-like catalyst for degradation of organic pollutants. Front. Environ. Sci. Eng. 13, 18 (2019). https://doi.org/10.1007/s11783-019-1101-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-019-1101-z