Abstract
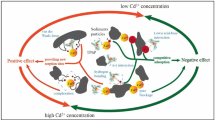
Batch sorption experiments were conducted to evaluate the sorption behavior of tetracycline (TC, H3L) on sediments and soils in the presence and absence of cadmium (Cd), as affected by pH and properties of sediments and soils. The results indicated stronger nonlinearity and higher capacity of TC sorption on sediments than on soils. Sorption of TC also strongly depended on environmental factors and sediment/soil properties. Lower pH facilitated TC sorption through a cation exchange mechanism, which also took place at pH values above 5.5, where TC existed as a zwitterion (H2L0) or anions (HL− and L2−). When pH was above 7, however, ligand-promoted dissolution of TC might occur due to TC weakening the Al-O bond of aluminum oxide and the Fe-O bond of iron oxide. Natural organic matter (NOM) plays a more important role in TC sorption than cation exchange capacity (CEC) and clay contents. The presence of Cd (II) increased TC sorption on both sediments and soils, which resulted from the decrease of equilibrium solution pH caused by Cd2+ exchange with H+ ions of sediment/soil surfaces. The increase of TC sorption was also related to the formation of TC-Cd complexes, where Cd2+ acted as a bridge between the sediment/soil and TC.
Similar content being viewed by others
References
Cahill J D, Furlong E T, Burkhardt M R, Kolpin D, Anderson L G. Determination of pharmaceutical compounds in surface- and ground-water samples by solid-phase extraction and high-performance liquid chromatography-electrospray ionization mass spectrometry. Journal of Chromatography A, 2004, 1041(1–2): 171–180
Miao X S, Bishay F, Chen M, Metcalfe C D. Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environmental Science & Technology, 2004, 38(13): 3533–3541
Sarmah A K, Meyer M T, Boxall A B A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere, 2006, 65(5): 725–759
Zhu J, Snow D D, Cassada D A, Monson S J, Spalding R F. Analysis of oxytetracycline, tetracycline, and chlortetracycline in water using solid-phase extraction and liquid chromatography-tandem mass spectrometry. Journal of Chromatography. A, 2001, 928(2): 177–186
Yang S, Cha J, Carlson K. Quantitative determination of trace concentrations of tetracycline and sulfonamide antibiotics in surface water using solid-phase extraction and liquid chromatography/ion trap tandem mass spectrometry. Rapid Communications in Mass Spectrometry: RCM, 2004, 18(18): 2131–2145
Ben W W, Qiang Z M, Adams C, Zhang H Q, Chen L P. Simultaneous determination of sulfonamides, tetracyclines and tiamulin in swine wastewater by solid-phase extraction and liquid chromatography-mass spectrometry. Journal of Chromatography. A, 2008, 1202(2): 173–180
Kim S, Eichhorn P, Jensen J N, Weber A S, Aga D S. Removal of antibiotics in wastewater: effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environmental Science & Technology, 2005, 39(15): 5816–5823
Kemper N. Veterinary antibiotics in the aquatic and terrestrial environment. Ecological Indicators, 2008, 8(1): 1–13
Kim S C, Carlson K. Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environmental Science & Technology, 2007, 41(1): 50–57
Figueroa R A, Leonard A, MacKay A A. Modeling tetracycline antibiotic sorption to clays. Environmental Science & Technology, 2004, 38(2): 476–483
Kulshrestha P, Giese R F Jr, Aga D S. Investigating the molecular interactions of oxytetracycline in clay and organic matter: insights on factors affecting its mobility in soil. Environmental Science & Technology, 2004, 38(15): 4097–4105
Figueroa R A, MacKay A A. Sorption of oxytetracycline to iron oxides and iron oxide-rich soils. Environmental Science & Technology, 2005, 39(17): 6664–6671
Sassman S A, Lee L S. Sorption of three tetracyclines by several soils: assessing the role of pH and cation exchange. Environmental Science & Technology, 2005, 39(19): 7452–7459
Pils J R V, Laird D A. Sorption of tetracycline and chlortetracycline on K- and Ca-saturated soil clays, humic substances, and clay-humic complexes. Environmental Science & Technology, 2007, 41(6): 1928–1933
Xu X R, Li X Y. Sorption and desorption of antibiotic tetracycline on marine sediments. Chemosphere, 2010, 78(4): 430–436
Wessels J M, Ford W E, Szymczak W, Schneider S. The complexation of tetracycline and anhydrotetracycline with Mg2+ and Ca2+: a spectroscopic study. Journal of Physical Chemistry B, 1998, 102(46): 9323–9331
Wang Y J, Jia D A, Sun R J, Zhu H W, Zhou D M. Adsorption and cosorption of tetracycline and copper(II) on montmorillonite as affected by solution pH. Environmental Science & Technology, 2008, 42(9): 3254–3259
Jia D A, Zhou D M, Wang Y J, Zhu H W, Chen J L. Adsorption and cosorption of Cu (II) and tetracycline on two soils with different characteristics. Geoderma, 2008, 146(1–2): 224–230
Eurola M, Hietaniemi V, Kontturi M, Tuuri H, Pihlava J M, Saastamoinen M, Rantanen O, Kangas A, Niskanen M. Cadmium contents of oats (Avena sativa L.) in official variety, organic cultivation, and nitrogen fertilization trials during 1997–1999. Journal of Agricultural and Food Chemistry, 2003, 51(9): 2608–2614
Tu Y J, Han X Y, Xu Z R, Wang Y Z, Li W F. Effect of cadmium in feed on organs and meat colour of growing pigs. Veterinary Research Communications, 2007, 31(5): 621–630
Nicholson F A, Smith S R, Alloway B J, Carlton-Smith C, Chambers B J. An inventory of heavy metals inputs to agricultural soils in England and Wales. The Science of the Total Environment, 2003, 311(1–3): 205–219
Azeez J O, Adekunle I O, Atiku O O, Akande K B, Jamiu-Azeez S O. Effect of nine years of animal waste deposition on profile distribution of heavy metals in Abeokuta, south-western Nigeria and its implication for environmental quality. Waste Management (New York, NY), 2009, 29(9): 2582–2586
Li Y K. Soil Agrochemistry Analysis. Beijing: Science Press, 1984
Zhang J H, Zeng J H, He M C. Effects of temperature and surfactants on naphthalene and phenanthrene sorption by soil. Journal of Environmental Sciences (China), 2009, 21(5): 667–674
Rabølle M, Spliid N H. Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere, 2000, 40(7): 715–722
Zhou D M, Wang Y J, Cang L, Hao X Z, Luo X S. Adsorption and cosorption of cadmium and glyphosate on two soils with different characteristics. Chemosphere, 2004, 57(10): 1237–1244
Zhuang J, Yu G R. Effects of surface coatings on electrochemical properties and contaminant sorption of clay minerals. Chemosphere, 2002, 49(6): 619–628
Jones A D, Bruland G L, Agrawal S G, Vasudevan D. Factors influencing the sorption of oxytetracycline to soils. Environmental Toxicology and Chemistry/SETAC, 2005, 24(4): 761–770
Gu C, Karthikeyan K G. Interaction of tetracycline with aluminum and iron hydrous oxides. Environmental Science & Technology, 2005, 39(8): 2660–2667
Gu C, Karthikeyan K G, Sibley S D, Pedersen J A. Complexation of the antibiotic tetracycline with humic acid. Chemosphere, 2007, 66(8): 1494–1501
Lai C H, Chen C Y, Wei B L, Yeh S H. Cadmium adsorption on goethite-coated sand in the presence of humic acid. Water Research, 2002, 36(20): 4943–4950
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, G., Liu, X., Sun, K. et al. Sorption of tetracycline to sediments and soils: assessing the roles of pH, the presence of cadmium and properties of sediments and soils. Front. Environ. Sci. Eng. China 4, 421–429 (2010). https://doi.org/10.1007/s11783-010-0265-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-010-0265-3