Abstract
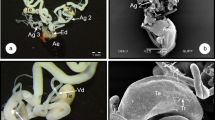
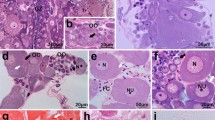
In this study, oocyte development and vitellogenesis of the leaf beetle Chrysomela populi are described and illustrated using light and scanning electron microscopies. The female reproductive tract of C. populi consists of a pair of ovaries, a pair of lateral oviducts, a common oviduct and a spermathecae. Each ovary has 14 telotrophic meroistic ovarioles. Each ovariole consists of a terminal filament, germarium, vitellarium and pedicel. Terminal filament is thin, long thread shaped. The germarium contains trophocytes towards the distal part, prefollicular cells and oocytes towards the proximal part in the young stage. The vitellarium contains 3–4 oocytes at different stages of development (previteloogenic, vitellogenic and choriogenic oocytes). Previtellogenic oocytes located towards the distal end of the vitellarium are surrounded by follicle epithelium. Vitellogenic oocytes are surrounded by a monolayer follicular epithelium, and their ooplasma is basophilic. The ooplasma of the oocytes in the choriogenic stage, which is the last stage of vitellogenesis, is filled with eosinophilic yolk granules of different sizes. Chorion is clearly differentiated between the vitelline membrane surrounding the ooplasma and the follicular epithelium. The pedicel connects the ovarioles with the lateral oviduct. A pair of lateral oviducts are connected to the common oviduct. A spermatheca is located towards the proximal part of the common oviduct. Spermatheca consists of a reservoir, duct and gland. The eggs are light yellow in color and the chorion is very thin. This study intends to contribute to female reproductive biology studies in Coleoptera and other insect order species, agricultural struggle studies in terms of researching C. populi.






Similar content being viewed by others
References
Aslan I, Özbek H (1999) Faunistic and systematic studies on the subfamily Chrysomelinae (Coleoptera, Chrysomelidae) in Artvin, Erzincan and Erzurum provinces of Turkey. Turk J Zool 23:751–767
Aslan GE, Kaya ÖD, Ünal E (2020) Contributions to the knowledge of leaf beetle (Coleoptera: Chrysomelidae) fauna in Elazığ, Erzincan and Tunceli provinces. Journal of Graduate School of Natural and Applied Sciences of Mehmet Akif Ersoy University 11(Suppl Issue 1):273–280. https://doi.org/10.29048/makufebed.783029
Assis MQ, Dohanik VT, de Oliveira LL, Zanuncio JC, Serrão JE (2019) Evidence for a transcellular route for vitellogenin transport in the telotrophic ovary of Podisus nigrispinus (Hemiptera: Pentatomidae). Sci Rep 9(1):1–8. https://doi.org/10.1038/s41598-019-52789-z
Boonyoung P, Senarat S, Kettratad J, Jiraungkoorskul W, Thaochan N, Sing KW, Pengsakul P, Poolprasert P (2020) Mature gonadal histology and gametogenesis of the tortoise beetle Aspidimorpha sanctaecrucis (Fabricius, 1792) (Coleoptera: Cassidinae: Chrysomelidae): Histological observation. Songklanakarin J Sci Technol 42(4):725–947
Büning J (1994) The insect ovary: ultrastructure, previtellogenic growth and evolution. First edn. Chapman & Hall, London
Caron E, Ribeiro-Costa CS, Linzmeier AM (2004) The egg morphology of some species of Sennius Bridwell (Coleoptera: Chrysomelidae: Bruchinae) based on scanning electron micrographs. Zootaxa 556(1):1–10
Cavalcaselle B (1972) Pilot experiments for the control of insect pests of poplar in the nursery by means of systemic insecticides in granular form. Celulosa e Carta 23:17–25
Cheetham T (1992) Anatomy and histology of tissues associated with egg deposition in Chrysomela scripta Fabr. (Coleoptera: Chrysomelidae). J Kans Entomol Soc 65(2):101–114
Devasahayam S, Vidyasagar PSPV, Koya KM (1998) Reproductive system of pollu beetle, Longitarsus nigripennis Motschulsky (Coleoptera: Chrysomelidae), a major pest of black pepper, Piper nigrum Linnaeus. J Entomol Res 22(1):77–82
Georgiev G (2000) Species composition and impact of the phytophagous insects on the poplars (Populus spp.) in Bulgaria. Nauka za Gorata 37:45–54
Gerber GH, Neill GB, Westdal PH (1978) The anatomy and histology of the internal reproductive organs of the sunflower beetle, Zygogramma exclamationis (Coleoptera: Chrysomelidae). Can J Zool 56(12):2542–2553
Goldson SL, Emberson RM (1981) Reproductive morphology of the Argentine stem weevil, Hyperodes bonariensis (Coleoptera: Curculionidae). N Z J Zool 8(1):67–77
Izumi S, Yano K, Yamamoto Y, Takahashi SY (1994) Yolk proteins from insect eggs: structure, biosynthesis and programmed degradation during embryogenesis. J Insect Physiol 40:735–746
Jolivet P (2015) Together with 30 years of symposia on Chrysomelidae! Memories and personal reflections on what we know more about leaf beetles. In: Jolivet P, Santiago-Blay J, Schmitt M (eds) Research on Chrysomelidae 5. Zookeys, pp 35–61
Jolivet P, Petipierre E, Hasiao TH (1988) Biology of chrysomelidae. Series entomologica, 42, Kluver Academic Puplishers, Dordrecht
Kasap H (1988) A list of some Chrysomelinae (Col., Chrysomelidae) from Turkey. II. Türk Entomol Derg 12:85–95
Khan MA, Ahmad D (1991) Comparative toxicity of some chlorinated hydrocarbon insecticides to poplar leaf beetle–Chrysomela populi L. (Coleoptera: Chrysomelidae). Indian J For 14:42–45
Klowden MJ (2009) Spermatheca. In: Vincent HR, Ring TC (eds) Encyclopedia of insects, 2nd edn. Academic, Burlington, pp 939–940
Konstantinov AS, Korotyaev BA, Volkovitsh MG (2009) Insect biodiversity in the Palearctic region. In: Foottit RG, Adler PH (eds) Insect biodiversity: science and society. Wiley Blackwell, Oxford, pp 107–162
Kumar R, Attah PK (1977) Structure of the organs of digestion and reproduction in the Tortoise Beetle, Aspidomorpha spp. (Chrysomelidae: Coleoptera). J Nat Hist 11(1):65–76
Maffei EMD, Fragoso DDB, Pompolo SDG, Serrão JE (2001) Morphological and cytogenetical studies on the female and male reproductive organs of Eriopis connexa Mulsant (Coleoptera, Polyphaga, Coccinellidae). Neth J Zool 51(4):483–496
Marzo L (2008) Studio sulla diversità anatomica della spermateca nei Coleotteri. Entomologica 41:13–76
Melo ACA, Valle D, Machado EA, Salerno AP, Paiva-Silva GO, Silva NLCE, de Souza W, Masuda H (2000) Synthesis of vitellogenin by the follicle cells of Rhodnius prolixus. Insect Biochem Mol Biol 30:549–557
Metcalfe ME (1932) Memoirs: the structure and development of the reproductive system in the Coleoptera with notes on its homologies. J Cell Sci 2(297):49–129
Mohamed MI, Khaled AS, Fattah HMA, Hussein MA, Salem DA, Fawki S (2015) Ultrastructure and histopathological alteration in the ovaries of Callosobruchus maculatus (F.) (Coleoptera, Chrysomelidae) induced by the solar radiation. J Basic Appl Zool 68:19–32. https://doi.org/10.1016/j.jobaz.2014.12.004
Nation JL (1983) A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. Stain Technol 58(6):347–351
Putman WL (1955) Bionomics of Stethorus punctillum Weise (Coleoptera: Coccinellidae) in Ontario. Can Entomol 87:9–33
Raikhel AS, Dhadialla TS (1992) Accumulation of yolk proteins in insect oocytes. Annu Rev Entomol 37:217–251. https://doi.org/10.1146/annurev.en.37.010192.001245
Salazar K, Boucher S, Serrão JE (2017) Structure and ultrastructure of the ovary in the South American Veturius sinuatus (Eschscholtz) (Coleoptera, Passalidae). Arthropod Struct Dev 46(4):613–626. https://doi.org/10.1016/j.asd.2017.03.007
Simões MV (2012) Male and female reproductive systems of Stolas conspersa (Germar) (Coleoptera, Chrysomelidae, Cassidinae). Rev Bras Entomol 56(1):19–22. https://doi.org/10.1590/S0085-56262012005000007
Stringer IAN (1988) The female reproductive system of Costelytra zealandica (White) (Coleoptera: Scarabaeidae: Melolonthinae). N Z J Zool 15(4):513–533
Świątek P, Górkiewicz T (2006) Microsporidia infect the Liophloeus lentus (Insecta, Colepotera) ovarioles, developing oocytes and eggs. Fol Biol 54(1–2):61–67. https://doi.org/10.3409/173491606777919120
Thakur ML (1999) Insect pest status of poplars in India. Indian For 125:866–872
Tillesse V, Nef L, Charles J, Hopkin A, Augustin S (2007) Damaging poplar ınsects (ınternationally ımportant species). International Poplar Commission, FAO, Rome
Túler AC, Silva Torres CS, Teixeira VW, Teixeira AA, Guedes CA, D’Assunção CG, Brayner FA, Alves LC (2018) Histology of the spermateca and stored sperm of Tenuisvalvae notata (Coleoptera: Coccinellidae). Physiol Entomol 43(3):180–187. https://doi.org/10.1111/phen.12242
Urban J (2006) Occurrence, bionomics and harmfulness of Chrysomela populi L. (Coleoptera; Chrysomelidae). J For Sci 52:255–284
Wan Nurul ‘Ain WMN, Nurul Wahida O, Yaakop S, Norefrina Shafinaz MN (2018) Morphology and histology of reproductive organ and first screening of Wolbachia in the ovary of red palm weevil, Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae). Serangga 23(2):183–193
Wang XP, Zhou XM, Wang YY, Lei CL (2007) Internal reproductive system and diapausing morphology of the Brassica leaf beetle Phaedon brassicae Baly (Coleoptera: Chrysomelidae: Chrysomelinae). Coleopt Bull 61(3):457–462
Wheeler DE (2009) Reproduction, female. In: Vincent HR, Ring TC (eds) Encyclopedia of insects, 2nd edn. Academic, California, pp 183–201
Wheeler DE (2009) Ovarioles. In: Vincent HR, Ring TC (eds) Encyclopedia of insects, 2nd edn. Academic, California, pp 743–744
Wigthman JA, Southgate BJ (1982) Egg morphology, host, and probable regions of origin of the bruchids (Coleoptera: Bruchidae) that infest stored pulses-an identification aid. New Zeal J Exp Agr 10:95–99
Zeki H, Toros S (1996) The effect of host on the adults of Chrysomela populi L. and Chrysomela tremulae F. (Col:Chrysomelidae). Bitki Kor Bült 36:25–38
Zhang Z (2011) Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa 3148:1–237
Zhang SQ, Che LH, Li Y, Liang D, Pang H, Ślipiński A, Zhang P (2018) Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat Commun 9(1):1–11. https://doi.org/10.1038/s41467-017-02644-4
Zimowska G, Shirk PD, Silhacek DL, Shaaya E (1994) Yolk sphere formation is initiated in oocytes before development of patency in follicles of the moth Plodia interpunctella. Wilhelm Roux’s Arch Dev Biol 203:215–226
Acknowledgements
We thank Gazi University Academic Writing Center for revising the grammar of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest disclosed in this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Addıtıonal informatıon
This article was originally submitted by the authors in English and is first published here.
Rights and permissions
About this article
Cite this article
Özyurt Koçakoğlu, N., Candan, S. & Güllü, M. Morphology of the reproductive tract of females of leaf beetle Chrysomela populi (Chrysomelidae: Coleoptera). Biologia 76, 3257–3265 (2021). https://doi.org/10.1007/s11756-021-00796-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00796-9