Abstract
Objectives
Atrial natriuretic peptide is a cardiac atrium-derived hormone and its cardioprotective effects have recently been confirmed, but the actual mechanism underlying these effects has not been well elucidated. In this study, we proposed that atrial natriuretic peptide achieves its effects in part via peroxisome proliferator activated receptor γ, a nuclear receptor.
Methods
Hemodynamic data in swine heart ischemia–reperfusion model were measured under the conditions of no medication for control (Group N, n = 8) or that of carperitide (synthetic human atrial natriuretic peptide) systemic administration (Group A, n = 8). After 30 min of left anterior descending artery total occlusion and 4 h of reperfusion, peroxisome proliferator activated receptor γ mRNA and protein expressions in cardiac muscle were examined. The mRNA expression of Liver X receptor α, the downstream agent of peroxisome proliferator activated receptor γ, was also evaluated. Creatine kinase-myocardial band and Troponin T elevations after reperfusion were evaluated as markers of cardiac damage.
Results
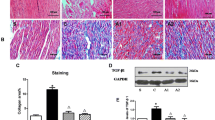
The dP/dT decrease during reperfusion was ameliorated in Group A. Peroxisome proliferator activated receptor γ mRNA expression in Group A was significantly higher in ischemic area than that in Group N, although the difference was not significant in the marginal and non-ischemic areas. The peroxisome proliferator activated receptor γ protein expression in ischemic area was also significantly dominant in Group A.
Conclusions
Atrial natriuretic peptide may achieve its cardioprotective effects in part via the activation of the peroxisome proliferator activated receptor γ pathway, particularly in central areas of ischemic lesions.







Similar content being viewed by others
References
Kangawa K, Matsuo H. Purification and complete amino acid sequence of alpha-human atrial natriuretic polypeptide (alpha-hANP). Biochem Biophys Res Commun. 1984;118:131–9.
Schlueter N, de Sterke A, Willmes DM, Spranger J, Jordan J, Birkenfeld AL. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol Ther. 2014;144:12–27.
Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–93.
Takagi G, Kiuchi K, Endo T, Yamamoto T, Sato N, Nejima J, et al. Alpha-human atrial natriuretic peptide, carperitide, reduces infarct size but not arrhythmias after coronary occlusion/reperfusion in dogs. J Cardiovasc Pharmacol. 2000;30:22–30.
Kishimoto I, Rossi K, Garbers DL. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci U S A. 2001;98:2703–6.
Tsuneyoshi H, Nishina T, Nomoto T, Kanemitsu H, Kawakami R, Unimonh O, et al. Atrial natriuretic peptide helps prevent late remodeling after left ventricular aneurysm repair. Circulation. 2004;110:II-174–9.
D’Souza SP, Davis M, Baxter GF. Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther. 2004;101:113–29.
Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–88.
Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARg and the global map of adipogenesis and beyond. Trends Endocrinol Metab. 2014;25:293–302.
Wakabayashi K, Okamura M, Tsutsumi S, Nishikawa NS, Tanaka T, Sakakibara I, et al. The peroxisome proliferator-activated receptor γ/retinoid x receptor α heterodimer targets the histone modification enzyme pr-set7/setd8 gene and regulates adipogenesis through a positive feedback loop. Mol Cell Biol. 2009;29:3544–55.
Olefsky JM, Saltiel AR. PPARγ and the treatment of insulin resistance. Trends Endocrinol Metab. 2000;11:362–8.
Abdelrahman M, Sivarajah A, Thiemermann C. Beneficial effects of PPAR-g ligands in ischemia–reperfusion injury, inflammation and shock. Cardiovasc Res. 2005;65:772–81.
Tao L, Wang Y, Gao E, Hangxiang Z, Yuexing Y, Wayne BL, et al. Adiponectin: an indispensable molecule in rosiglitazone cardioprotection following myocardial infarction. Circ Res. 2010;106:409–17.
Zhu P, Lu L, Xu Y, Schwartz GG. Troglitazone improves recovery of left ventricular function after regional ischemia in pigs. Circulation. 2000;101:1165–71.
Vollmar AM. The role of atrial natriuretic peptide in the immune system. Peptides. 2005;26:1086–94.
Heikkinen S, Auwerx J, Argmann CA. PPARgamma in human and mouse physiology. Biochim Biophys Acta. 2007;1771:999–1013.
Tsukamoto O, Fujita M, Kato M, Yamazaki S, Asano Y, Ogai A, et al. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 2009;53:2070–7.
Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–71.
Konishi T, Nakamura Y, Kato I, Kawai C. Dependence of peak dP/dt and mean ejection rate on load and effect of inotropic agents on the relationship between peak dP/dt and left ventricular developed pressure–assessed in the isolated working rat heart and cardiac muscles. Int J Cardiol. 1992;35:333–41.
Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–28.
Sangawa K, Nakanishi K, Ishino K, Inoue M, Kawada M, Sano S. Atrial natriuretic peptide protects against ischemia–reperfusion injury in the isolated rat heart. Ann Thorac Surg. 2004;77:233–7.
Okawa H, Horimoto H, Mieno S, Nomura Y, Yoshida M, Shinjiro S. Preischemic infusion of alpha-human atrial natriuretic peptide elicits myoprotective effects against ischemia reperfusion in isolated rat hearts. Mol Cell Biochem. 2003;248:171–7.
Wakui S, Sezai A, Tenderich G, Hata M, Osaka S, Taniguchi Y, et al. Experimental investigation of direct myocardial protective effect of atrial natriuretic peptide in cardiac surgery. J Thorac Cardiovasc Surg. 2010;139:918–25.
Gorbe A, Giricz Z, Szunyog A, Csont T, Burley DS, Baxter GF, et al. Role of cGMP-PKG signaling in the protection of neonatal rat cardiac myocytes subjected to simulated ischemia/reoxygenation. Basic Res Cardiol. 2010;105:643–50.
Lukowski R, Krieg T, Rybalkin SD, Beavo J, Hofmann F. Turning on cGMP-dependent pathways to treat cardiac dysfunctions: boom, bust, and beyond. Trends Pharmacol Sci. 2014;35:404–13.
Wang J, Yang K, Xu L, Zhang Y, Lai N, Jiang H, et al. Sildenafil inhibits hypoxia-induced transient receptor potential canonical protein expression in pulmonary arterial smooth muscle via cGMP-PKG-PPARg axis. Am J Respir Cell Mol Biol. 2012;49:231–40.
Taimor G, Hofstaetter B, Piper HM. Apoptosis induction by nitric oxide in adult cardiomyocytes via cGMP-signaling and its impairment after simulated ischemia. Cardiovasc Res. 2000;45:588–94.
Wu CF, Bishopric NH, Pratt RE. Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J Biol Chem. 1997;272:14860–6.
Hobson MJ, Hake PW, O’Connor M, Schulte C, Moore V, James JM, et al. Conditional deletion of cardiomyocyte peroxisome proliferator-activated receptor-γ enhances myocardial ischemia –reperfusion injury in mice. Shock. 2014;41:40–7.
Kuga H, Ogawa K, Oida A, Taguchi I, Nakatsugawa M, Hoshi T, et al. Administration of atrial natriuretic peptide attenuates reperfusion phenomena and preserves left ventricular regional wall motion after direct coronary angioplasty for acute myocardial infarction. Circ J. 2003;67:443–8.
Acknowledgments
This work was supported by MEXT KAKENHI Grant Number 15K10206. We wish to thank Ayako Ono and Teruko Sueta for assistance with the animal experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Rights and permissions
About this article
Cite this article
Suzuki, T., Saiki, Y., Horii, A. et al. Atrial natriuretic peptide induces peroxisome proliferator activated receptor γ during cardiac ischemia–reperfusion in swine heart. Gen Thorac Cardiovasc Surg 65, 85–95 (2017). https://doi.org/10.1007/s11748-016-0704-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-016-0704-6