Abstract
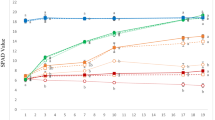
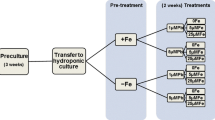
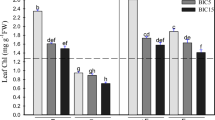
The study was aimed to determine the iron (Fe) requirements at different growing stages of pot marigold (Calendula officinalis L.). Plants were grown in pots containing equal proportions of sand and perlite, and fertigated with nutrient solution including 4 or 35 µM of the Fe-chelate Fe(III)-EDDHA in each of three different growth stages. Stages were labeled I (from seed germination until 4th to 6th true leaf stage), II (from 4th to 6th true leaf stage until start of flowering) and III (from the start of flowering to flower fading time). Using consistently 4 µM Fe led to chlorotic plants and small and short-lived flowers, whereas using consistently 35 µM Fe led to good quality flowers, considering size, number and lifetime. Results suggest that using 4 µM Fe at growth stages I or III did not result in significant reductions in flower weight and number. Conversely, using 4 µM Fe at stage II led to decreases in flower weight and number. Using 4 µM Fe in two stages could be acceptable only when stages were I and III, although some decreases in number of flowers and number longevity occurred. Results show that it is feasible to reduce the concentration of Fe in the nutrient solution during a considerable part of the plant growth period. Using low Fe in only one growth stage would reduce total Fe inputs by 20–29%, whereas using low Fe in stages I and III would reduce total Fe inputs by 48%. The reduction proposed in the Fe-chelate concentrations would result in diminishing costs for growers and decreasing the environmental impact of Fe fertilization.








Similar content being viewed by others
References
Abadía J, Tagliavini M, Grasa R, Belkhodja R, Abadía A, Sanz M, Faria EA, Tsipouridis C, Bruno M (2000) Using the flower Fe concentration for estimating chlorosis status in fruit tree orchards: a summary report. J Plant Nutr 23:2024–2033
Abadía J, Vázquez S, Rellán-Álvarez R, El-Jendoubi H, Abadía A, Álvarez-Fernández A, López-Millán AF (2011) Towards a knowledge-based correction of iron chlorosis. Plant Physiol Biochem 49:471–482
Adamski JM, Danieloski R, Deuner S, Braga EJ, de Castro LA, Peters JA (2012) Responses to excess iron in sweet potato: impacts on growth, enzyme activities, mineral concentrations, and anatomy. Acta Physiol Plant 34:1827–1836
Ahmad I, Khan MA, Qasim M, Ahmad R, Randhawa MA (2010) Growth, yield and quality of Rosa hybrida L. as influenced by various micronutrients. Pak J Agri Sci 47:5–12
Alaey M, Babalar M, Naderi R, Kafi M (2011) Effect of pre-and postharvest salicylic acid treatment on physio-chemical attributes in relation to vase-life of rose cut flowers. Postharvest Biol Technol 61:91–94
Álvarez-Fernández A, García-Marco S, Lucena JJ (2005) Evaluation of synthetic iron (III)-chelates (EDDHA/Fe3+, EDDHMA/Fe3+ and the novel EDDHSA/Fe3+) to correct iron chlorosis. Eur J Agron 22:119–130
Álvarez-Fernández A, Abadía J, Abadía A (2006) Iron deficiency, fruit yield and fruit quality. In: Abadia J (ed) Barton LL. Iron nutrition in plants and rhizospheric microorganisms, Springer, Dordrecht, pp 85–101
Amuamuha L, Pirzad A, Hadi H (2012) Effect of varying concentrations and time of nanoiron foliar application on the yield and essential oil of pot marigold. Int Res J Appl Basic Sci 3:2085–2090
Benton-Jones J, Wolf B, Mills HA (1991) Plant analysis handbook: a practical sampling, preparation, analysis, and interpretation guide. Micro-Macro-Publishing, Athens
Bhute P, Panchbhai D, Raut V, Neha C, Hemlata K (2017) Studies on flower production in annual chrysanthemum in response to iron and zinc. Plant Arch 17:1017–1019
Briat JF, Dubos C, Gaymard F (2015) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci 20:33–40
Castro E, Pinto J, Melo HCd, Soares A, Alvarenga AA, Lima Júnior E (2005) Aspectos anatômicos e fisiológicos de plantas de guaco submetidas a diferentes fotoperíodos. Hortic Bras 23:846–850
Chakraborty B, Singh PN, Shukla A, Mishra DS (2012) Physiological and biochemical adjustment of iron chlorosis affected low-chill peach cultivars supplied with different iron sources. Physiol Mol Biol Plants 18:141–148
Chance B, Maehly A (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Cheema MA, Malik MA, Hussain A, Shah SH, Basra SMA (2001) Effects of time and rate of nitrogen and phosphorus application on the growth and the seed and oil yields of canola (Brassica napus L.). J Agron Crop Sci 186:103–110
Corrêa Monteiro R, Brasil Pereira Pinto JE, Soares Reis É, de Oliveira C, de Castro EM, da Silva BR (2009) Características anatômicas foliares de plantas de orégano (Origanum vulgare L.) submetidas a diferentes fontes e níveis de adubação orgânica. Acta Sci Agron 31:439–444
Doheny-Adams T, Hunt L, Franks PJ, Beerling DJ, Gray JE (2012) Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos Trans R Soc B 367:547–555
El-Jendoubi H, Melgar JC, Álvarez-Fernández A, Sanz M, Abadía A, Abadía J (2011) Setting good practices to assess the efficiency of iron fertilizers. Plant Physiol Biochem 49:483–488
El-Jendoubi H, Vázquez S, Calatayud Á, Vavpetič P, Vogel-Mikuš K, Pelicon P, Abadía J, Abadía A, Morales F (2014) The effects of foliar fertilization with iron sulfate in chlorotic leaves are limited to the treated area. A study with peach trees (Prunus persica L. Batsch) grown in the field and sugar beet (Beta vulgaris L.) grown in hydroponics. Front Plant Sci 5:1–5
Fernández V, Eichert T, Del Río V, López-Casado G, Heredia-Guerrero JA, Abadía A, Heredia A, Abadía J (2008) Leaf structural changes associated with iron deficiency chlorosis in field-grown pear and peach: physiological implications. Plant Soil 311:161–172
Huang H, Hu CX, Tan Q, Hu X, Sun X, Bi L (2012) Effects of Fe-EDDHA application on iron chlorosis of citrus trees and comparison of evaluations on nutrient balance with three approaches. Sci Hortic 146:137–142
Iqbal N, Khan NA, Ferrante A, Trivellini A, Francini A, Khan M (2017) Ethylene role in plant growth, development and senescence: interaction with other phytohormones. Front Plant Sci 8:475
Kabir AH, Rahman MM, Haider SA, Paul NK (2015) Mechanisms associated with differential tolerance to Fe deficiency in okra (Abelmoschus esculentus Moench). Environ Exp Bot 112:16–26
Kim SH, Ahn YO, Ahn MJ, Lee HS, Kwak SS (2012) Down-regulation of β-carotene hydroxylase increases β-carotene and total carotenoids enhancing salt stress tolerance in transgenic cultured cells of sweetpotato. Phytochemistry 74:69–78
Kolar JS, Grewal HS (1994) Effect of split application of potassium on growth, yield and potassium accumulation by soybean. Fertil Res 39:217–222
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
López-Bellido L, López-Bellido RJ, Redondo R (2005) Nitrogen efficiency in wheat under rainfed Mediterranean conditions as affected by split nitrogen application. Field Crops Res 94:86–97
Lu Q, Jia D, Zhang Y, Dai X, He M (2014) Split application of potassium improves yield and end-use quality of winter wheat. Agron J 106:1411–1419
Lucena JJ, Hernández-Apaolaza L (2017) Iron nutrition in plants: an overview. Plant Soil 418:1–4
MacAdam JW, Nelson CJ, Sharp RE (1992) Peroxidase activity in the leaf elongation zone of tall fescue: I. Spatial distribution of ionically bound peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol 99:872–878
Marschner P (2012) Marschner’s mineral nutrition of higher plants. Academic Press, Boston
Melo HD, Castro ED, Soares A, Melo LD, Alves JD (2007) Alterações anatômicas e fisiológicas em Setaria anceps Stapf ex Massey e Paspalum paniculatum L. sob condições de déficit hídrico. Hoehnea 34:145–153
Memon SA, Abdul R, Muhammad A, Mahmooda B (2013) Effect of zinc sulphate and iron sulphate on the growth and flower production of gladiolus (Gladiolus hortulanus). J Agric Technol 9:1621–1630
Nenova VR (2009) Growth and photosynthesis of pea plants under different iron supply. Acta Physiol Plant 31:385
Pavlovic J, Samardzic J, Maksimović V, Timotijevic G, Stevic N, Laursen KH, Hansen TH, Husted S, Schjoerring JK, Liang Y (2013) Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol 198:1096–1107
Pestana M, de Varennes A, Abadía J, Faria EA (2005) Differential tolerance to iron deficiency of citrus rootstocks grown in nutrient solution. Sci Hortic 104:25–36
Pirzad A, Shokrani F (2012) Effects of iron application on growth characters and flower yield of Calendula officinalis L. under water stress. World Appl Sci J 18:1203–1208
Ribeiro TP, Fernandes C, Melo KV, Ferreira SS, Lessa JA, Franco RW, Schenk G, Pereira MD, Horn A Jr (2015) Iron, copper, and manganese complexes with in vitro superoxide dismutase and/or catalase activities that keep Saccharomyces cerevisiae cells alive under severe oxidative stress. Free Radic Biol Med 80:67–76
Rubinstein B (2000) Regulation of cell death in flower petals. Plant Mol Biol 44:303–318
Sanz M, Montañés L (1995) Flower analysis as a new approach to diagnosing the nutritional status of the peach tree. J Plant Nutr 18:1667–1675
Schmidt W, Tittel J, Schikora A (2000) Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol 122:1109–1118
Sudre D, Gutierrez-Carbonell E, Lattanzio G, Rellán-Álvarez R, Gaymard F, Wohlgemuth G, Fiehn O, Álvarez-Fernández A, Zamarreño AM, Bacaicoa E (2013) Iron-dependent modifications of the flower transcriptome, proteome, metabolome, and hormonal content in an Arabidopsis ferritin mutant. J Exp Bot 64:2665–2688
Sun B, Jing Y, Chen K, Song L, Chen F, Zhang L (2007) Protective effect of nitric oxide on iron deficiency-induced oxidative stress in maize (Zea mays). J Plant Physiol 164:536–543
Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK (2003) Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell 15:1263–1280
Terry N, Abadía J (1986) Function of iron in chloroplasts. J Plant Nutr 9:609–646
Ulger S, Sonmez S, Karkacier M, Ertoy N, Akdesir O, Aksu M (2004) Determination of endogenous hormones, sugars and mineral nutrition levels during the induction, initiation and differentiation stage and their effects on flower formation in olive. Plant Growth Regul 42:89–95
Vigani G, Zocchi G, Bashir K, Philippar K, Briat JF (2013) Signals from chloroplasts and mitochondria for iron homeostasis regulation. Trends Plant Sci 18:305–311
Vijay I, Nageswara Rao MB, Sudhavani V, Viji CP (2018) Effect of iron on vegetative growth, flowering, corm and cormel production in gladiolus (Gladiolus grandiflorus L.) cv. white prosperity. Int J Pure Appl Biosci 6:564–569
Wang S, Jiang J, Li T, Li H, Wang C, Wang Y, Liu G (2011) Influence of nitrogen, phosphorus, and potassium fertilization on flowering and expression of flowering-associated genes in white birch (Betula platyphylla Suk.). Plant Mol Biol Rep 29:794–880
Wójcik P, Filipczak J (2019) Prognosis of the nutritional status of apple trees based on prebloom leaves and flowers. J Plant Nutr. https://doi.org/10.1080/01904167.2019.1648683 (In press)
Yang G, Tang H, Nie Y, Zhang X (2011) Responses of cotton growth, yield, and biomass to nitrogen split application ratio. Eur J Agron 35:164–170
Yang G, Li J, Liu W, Yu Z, Shi Y, Lv B, Wang B, Han D (2015) Molecular cloning and characterization of MxNAS2, a gene encoding nicotianamine synthase in Malus xiaojinensis, with functions in tolerance to iron stress and misshapen flower in transgenic tobacco. Sci Hortic 183:77–86
Zanão Júnior LA, Carvalho-Zanão MP, Pereira N, Grossi JAS, Santos RF (2017) Iron doses in the production of potted rosebushes. Cienc Rural 47:1–6
Zhao Y, Chan Z, Gao J, Xing L, Cao M, Yu C, Hu Y, You J, Shi H, Zhu Y (2016) ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc Natl Acad Sci USA 113:1949–1954
Ziaeian AH, Malakouti MJ (2001) Effects of Fe, Mn, Zn and Cu fertilization on the yield and grain quality of wheat in the calcareous soils of Iran. In: Horst WJ et al (eds) Plant nutrition. developments in plant and soil sciences, vol 92. Springer, Dordrecht, pp 840–841
Acknowledgements
This research was financed by Lorestan University, Iran. JA was partially supported by the Spanish State Research Agency (project AGL2016-75226-R, AEI/FEDER, EU).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Esposito.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Izadi, Z., Rezaei Nejad, A. & Abadía, J. Physio-morphological and biochemical responses of pot marigold (Calendula officinalis L.) to split iron nutrition. Acta Physiol Plant 42, 6 (2020). https://doi.org/10.1007/s11738-020-3011-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-3011-x