Abstract
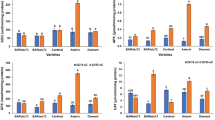
Drought stress is the main limiting factor of crop productivity. Wild soybean (Glycine soja) is a fine germplasm resource, which has a high tolerance to adverse environmental conditions. This study aimed to reveal the mechanism responsible for drought tolerance in drought-tolerant wild soybean. Here, the growth parameters and metabolomics of the two wild soybean varieties’ seedlings were analyzed under polyethylene glycol-simulated drought stress using gas chromatography–mass spectrometry. In total, 61 differentially accumulated metabolites were identified in leaves under polyethylene glycol-6000-simulated drought conditions. Compared with common wild soybean, the drought-tolerant wild soybean grew better. A metabolite profiling analysis suggested that the tricarboxylic acid cycle was enhanced in drought-tolerant wild soybean but inhibited in common wild soybean compared with the control group under simulated drought stress. Thus, the accumulation of osmotic compounds and the enhancement of energy and secondary antioxidant metabolism under drought-stress conditions are the mechanisms responsible for drought tolerance in drought-tolerant wild soybean. The results provide an important theoretical basis for utilizing wild soybean resources.




Similar content being viewed by others
References
Adamski J, Suhre K (2013) Metabolomics platforms for genome wide association studies-linking the genome to the metabolome. Curr Opin Biotech 24:39–47
Ahmed IM, Nadira UA, Bibi N, Cao F, He X, Zhang G, Wu F (2015) Secondary metabolism and antioxidants are involved in the tolerance to drought and salinity, separately and combined, in Tibetan wild barley. Environ Exp Bot 111:1–12
Araujo WL, Nunes-Nesi A, Nikoloski Z, Sweetlove LJ, Fernie AR (2012) Metabolic control and regulation of the tricarboxylic acid cyclein photosynthetic and heterotrophic plant tissues. Plant Cell Environ 35:1–21
Ashraf M, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Ashrafi M, Azimi-Moqadam MR, Moradi P, MohseniFard E, Shekari F, Kompany-Zareh M (2018) Effect of drought stress on metabolite adjustments in drought tolerant and sensitive thyme. Plant Physiol Bioch 132:391–399
Baxter A, Mittler R, Suzuki N (2014) Ros as key players in plant stress signalling. J Exp Bot 65:1229–1240
Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7:1099–1111
Bouche N, Fromm H (2004) GABA in plants: just a metabolite? Trends Plant Sci 9:110–115
Bourgaud F, Gravot A, Milesi S, Gontier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161:839–851
Chaves MM, Flexas J, Pinheiro C (2008) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chen Q, Tao SY, Bi XH, Xu X, Wang LL, Li XM (2013) Research progress in physiological and molecular biology mechanism of drought resistance in rice. Am J Mol Biol 3:102–107
Chiang PK, Gordon RK, Tal J, Zeng GC, Doctor BP, Pardhasaradhi K, McCann PP (1996) S-adenosylmethionine and methylation. FASEB J 10:471–480
Corso M, Vannozzi A, Maza E, Vitulo N, Meggio F, Pitacco A, Telatin A, D’Angelo M, Feltrin E, Negri AS, Prinsi B, Valle G, Ramina A, Bouzayen M, Bonghi C, Lucchin M (2015) Comprehensive transcript profiling of two grapevine rootstock genotypes contrasting in drought susceptibility links the phenylpropanoid pathway to enhanced tolerance. J Exp Bot 66:5739–5752
Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K (2011) Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biol 11:1–14
Edreva A, Velikova V, Tsonev T, Dagnon S, Gürel A, Akta SL, Gesheva E (2008) Stress-protective role of secondary metabolites: diversity of functions and mechanisms. Gen Appl Plant Physiol 34:67–78
Ekmekçi Y, Tanyolaç D, Ayhan B (2009) A crop tolerating oxidative stress induced by excess lead: maize. Acta Physiol Plant 31:319–330
Furlan AL, Bianucci E, Castro S, Dietz KJ (2017) Metabolic features involved in drought stress tolerance mechanisms in peanut nodules and their contribution to biological nitrogen fixation. Plant Sci 263:12
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Grierson CS, Barnes SR, Chase MW, Clarke M, Grierson D, Edwards KJ, Jellis GJ, Jones JD, Knapp S, Oldroyd G, Poppy G, Temple P, Williams R, Bastow R (2011) One hundred important questions facing plant science research. New Phytol 192:6–12
Guo R, Shi LX, Jiao Y, Li MX, Zhong XL, Gu FX, Liu Q, Xia X, Li HR (2018) Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 10:2
Hein JA, Sherrard ME, Manfredi KP, Abebe T (2016) The fifth leaf and spike organs of barley (Hordeum vulgare L.) display different physiological and metabolic responses to drought stress. BMC Plant Biol 16:248
Hura T, Hura K, Dziurka K, Ostrowska A, Baczek-Kwinta R, Grzesiak M (2012) An increase in the content of cell wall-bound phenolics correlates with the productivity of triticale under soil drought. J Plant Physiol 169:1728–1736
Jiao Y, Bai ZZ, Xu JY, Zhao ML, Khan Y, Hu YJ, Shi LX (2018) Metabolomics and its physiological regulation process reveal the salt-tolerant mechanism in glycine soja seedling roots. Plant Physiol Bioch 126:187
Khan N, Bano A, Rahman MA, Rathinasabapathi B, Babar MA (2018) Uplc-hrms based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ 42:115–132
Kingsbury RW, Epstein E, Pearcy RW (1984) Physiological responses to salinity inselected lines of wheat. Plant Physiol 74:417–423
Kocsy G, Laurie R, Szalai G, Szilágyi V, Simon-Sarkadi L, Galiba G, Jacoba A, Ronde D (2010) Genetic manipulation of proline levels affects antioxidants in soybean subjected to simultaneous drought and heat stresses. Physiol Plantarum 124:227–235
Lam HM, Xu X, Liu X, Chen WB, Yang GH, Wong FL, Li MW, He WM, Qin N, Wang B, Li J, Jian M, Wang J, Shao GH, Sun SSM, Zhang GY (2010) Resequencing of 31 wild and cultivated soybean genomes identifes patterns of genetic diversity and selection. Nat Genet 42:1053–1059
Less H, Galili G (2008) Principal transcriptional programs regulating plant amino acid metabolism in response to abiotic stresses. Plant Physiol 147:316–330
Li MX, Guo R, Jiao Y, Jin XF, Zhang HY, Shi LX (2017) Comparison of salt tolerance in soja based on metabolomics of seedling roots. Front Plant Sci 8:1101
Li MX, Xu JS, Wang XX, Fu H, Zhao ML, Wang H, Shi LX (2018) Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. J Plant Physiol 229:132–141
Marcinska I, Czyczyło-Mysza I, Skrzypek E, Filek M, Grzesiak S, Grzesiak MT, Janowiak F, Hura T, Dziurka M, Dziurka K, Nowakowska A, Quarrie SA (2013) Impact of osmotic stress on physiological and biochemical characteristics in drought susceptible and drought-resistant wheat genotypes. Acta Physiol Plant 35:451–461
Mejri M, Siddique KHM, Saif T, Abdelly C, Hessini K (2016) Comparative effect of drought duration on growth, photosynthesis, water relations, and solute accumulation in wild and cultivated barley species. J Plant Nutr Soil Sci 179:327–335
Mibei EK, Owino WO, Ambuko J, Giovannoni JJ, Onyango AN (2017) Metabolomic analyses to evaluate the effect of drought stress on selected African eggplant accessions. J Sci Food Agr 98:205–216
Mikami K, Murata N (2003) Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog Lipid Res 42:527–543
Moschen S, Rienzo JAD, Higgins J, Tohge T, Watanabe M, González S, Rivarola M, García-García F, Dopazo J, Esteban HH, Hoefgen R, Fernie AR, Paniego N, Fernández P, Heinz RA (2017) Integration of transcriptomic and metabolic data reveals hub, transcription factors involved in drought stress response in sunflower (Helianthus annuus L.). Plant Mol Biol 94:1–16
Nakabayashi R, Yonekurasakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, Michael AJ, Tohge T, Yamazaki M, Saito K (2014) Enhancement of oxidative and drought tolerance in arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77:367–379
Omidi H, Shams H, Sahandi MS, Rajabian T, Miransari M (2018) Balangu (Lallemantia sp.) growth and physiology under field drought conditions affecting plant medicinal content. Plant Physiol Bioch 130:641–646
Patel TK, Williamson JD (2016) Mannitol in plants, fungi, and plant-fungal interactions. Trends Plant Sci 21:486–497
Piasecka A, Sawikowska A, Kuczyńska A, Ogrodowicz P, Mikołajczak K, Krystkowiak K, Gudys K, Guzy-Wrobelska J, Krajewski P, Kachlicki P (2017) Drought-related secondary metabolites of barley (Hordeum vulgare L.) leaves and their metabolomic quantitative trait loci. Plant J 89:898–913
Rabara RC, Tripathi P, Rushton PJ (2014) The potential of transcription factor-based genetic engineering in improving crop tolerance to drought. OMICS J Integr Biol 18:601–614
Rabara RC, Tripathi P, Rushton PJ (2017) Comparative metabolome profile between tobacco and soybean grown under water-stressed conditions. Biomed Res Int 2017:3065251
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulphonium compounds in higher plants. Annu Rev Plant Biol 44:357–383
Ronde JAD, Mescht AVD, Steyn HSF (2000) Proline accumulation in response to drought and heat stress in cotton. Afr Crop Sci J 8:85–91
Saito K, Matsuda F (2010) Metabolomics for functional genomics, systems biology, and biotechnology. Annu Rev Plant Biol 61:463–489
Schmidhuber J, Tubiello FN (2007) Global food security under climate change. Proc Natl Acad Sci USA 104:19703–19708
Shao S, Li MX, Yang DS, Zhang J, Shi LX (2016) The physiological variations of adaptation mechaniam in Glycine soja seedlings under saline and alkaline stresses. Pak J Bot 48:2183–2193
Shi HT, Ye TT, Song B, Qi XQ, Chan ZL (2015) Comparative physiological and metabolomic responses of four brachypodium distachyon, varieties contrasting in drought stress resistance. Acta Physiol Plant 37:122
Sicher RC, Timlin D, Bailey B (2012) Responses of growth and primary metabolism of water-stressed barley roots to rehydration. J Plant Physiol 169:686–695
Silvente S, Sobolev AP, Lara M (2012) Metabolite adjustments in drought tolerant and sensitive soybean genotypes in response to water stress. Plos One 7:e38554
Sun CX, Gao XX, Fu JQ, Zhou JH, Wu XF (2015) Metabolic response of maize (Zea mays L.) plants to combined drought and salt stress. Plant Soil 388:99–117
Tardieu F, Tuberosa R (2010) Dissection and modelling of abiotic stress tolerance in plants. Curr Opin Plant Biol 13:206–212
Ullah N, Yüce M, Gökçe ZNÖ, Budak H (2017) Comparative metabolite profiling of drought stress in roots and leaves of seven triticeae species. BMC Genomics 18:969
Urano K, Maruyama K, Ogata Y, Morishita Y, Takeda M, Sakurai N, Suzuki H, Saito K, Shibata D, Kobayashi M, Yamaguchi-Shinozaki K, Shinozaki K (2009) Characterization of the aba-regulated global responses to dehydration in arabidopsis by metabolomics. Plant J 57:1065–1078
Warth B, Parich A, Bueschl C, Schoefbeck D, Neumann NKN, Kluger B, Schuster K, Krska R, Adam G, Lemmens M, Schuhmacher R (2015) GC–MS based targeted metabolic profiling identifies changes in the wheat metabolome following deoxynivalenol treatment. Metabolomics 11:722–738
Wu DZ, Cai SG, Chen MX, Ye LZ, Chen ZH, Zhang HT, Dai F, Wu FB, Zhang GP (2013) Tissue metabolic responses to salt stress in wild and cultivated barley. Plos One 8:e55431
Wu XJ, Cai KF, Zhang GP, Zeng FR (2017) Metabolite profiling of barley grains subjected to water stress: to explain the genotypic difference in drought-induced impacts on malting quality. Front Plant Sci 8:1547
Xue ZC, Zhao SJ, Gao HY, Sun S (2014) The salt resistance of wild soybean (Glycine soja Sieb. et Zucc. ZYD 03262) under NaCl stress is mainly determined by Na+ distribution in the plant. Acta Physiol Plant 36:61–70
Yang DS, Zhang J, Li MX, Shi LX (2017) Metabolomics analysis reveals the salt-tolerant mechanism in glycine soja. J Plant Growth Reg 36:1–12
Yang LM, Fountain JC, Ji P, Ni X, Chen S, Lee RD, Kemerait RC, Guo B (2018) Deciphering drought-induced metabolic responses and regulation in developing maize kernels. Plant Biotechnol J 16:1616–1628
Yi XP, Zhang YL, Yao HS, Luo HH, Gou L, Chow WS, Zhang WF (2016) Rapid recovery of photosynthetic rate following soil water deficit and re-watering in cotton plants (Gossypium herbaceum L.) is related to the stability of the photosystems. J Plant Physiol 194:23–34
Yokoi S, Bressan RA, Hasegawa PM (2002) Salt stress tolerance of plants. JIRCAS Working Report, pp 25–33
Yu TF, Xu ZS, Guo JK, Wang YX, Abernathy B, Fu JD, Chen X, Zhou YB, Chen M, Ye XG, Ma YZ (2017) Improved drought tolerance in wheat plants overexpressing a synthetic bacterial cold shock protein gene secspa. Sci Rep 7:44050
Zhang M, Barg R, Yin MG, Gueta-Dahan Y, Leikin-Frenkel A, Salts Y, Shabtai S, Ben-Hayyim G (2010) Modulated fatty acid desaturation via overexpression of two distinct omega-3 desaturases differentially alters tolerance to various abiotic stresses in transgenic tobacco cells and plants. Plant J 44:361–371
Zhang J, Yang DS, Li MX, Shi LX (2016) Metabolic profiles reveal changes in wild and cultivated soybean seedling leaves under salt stress. Plos One 11:e0159622
Zungia GE, Fernandez J, Cristi R, Alberdi M, Corcuera LJ (1990) Lipid changes in barley seedlings subjected to water and cold stress. Phytochemistry 29:3087–3090
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 31870278) and the Collaborative Innovation of Scientific and Technological of Chinese Academy of Agricultural Sciences. We thank Jilin Academy of Agriculture Science for helping. We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by M. Stobiecki.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11738_2019_2939_MOESM1_ESM.docx
The following additional information is available in the online version of this article—Online Resource 1 Total ion current gas chromatography–mass spectrometry chromatograms of leaf extracts from two wild soybean varieties (DOCX 621 kb)
11738_2019_2939_MOESM2_ESM.docx
The following additional information is available in the online version of this article—Online Resource 2 The contributions of metabolites from two wild soybean varieties’ seedling leaves to the first principal component (PC1) and the second principal component (PC2) (DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Guo, R., Li, M. et al. Metabolomics reveals the drought-tolerance mechanism in wild soybean (Glycine soja). Acta Physiol Plant 41, 161 (2019). https://doi.org/10.1007/s11738-019-2939-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2939-1