Abstract
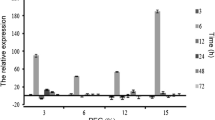
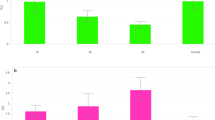
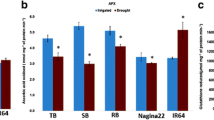
Drought is a major constraint of agriculture development. The intergeneric somatic hybrids between Brassica napus and Sinapis alba were created by electrofusion to obtain materials with enhanced drought tolerance. The drought tolerance of B. napus cv. Yangyou 6 (Y6) and one offspring line (W146) of the somatic hybrids was evaluated by morphological observation. Physiological parameters were determined in this study. Moreover, the activities of a few antioxidant enzymes and the transcript level of the antioxidant enzyme encoding genes were analyzed by qPCR. W146 and Y6 showed apparent wilting after drought stress for 7 days. However, Y6 wilted more severely than W146. The result of the physiological analysis showed that the relative electronic conductivity and malondialdehyde content of Y6 were higher than that of W146. The relative water content, net photosynthesis rate, proline content, and the activities of superoxide dismutase (SOD) and peroxidase in W146 were higher than that in Y6 after drought stress for 12 days. The DNA and nitrotetrazolium blue chloride staining analysis revealed less accumulation of O2 ·− and H2O2 in W146 than that in Y6 after drought stress. Moreover, the transcript level of some antioxidant enzyme encoding genes, such as Cu/ZnSOD, MnSOD, ascorbate peroxidase, glutathione reductase, and glutathione peroxidase in W146, was higher than that in Y6 under drought stress. Results revealed that line W146 showed more drought stress tolerance than Y6 because line W146 could reduce oxidative damage by efficient antioxidant systems.




Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- AsA:
-
Ascorbate
- AsA–GSH:
-
Ascorbate–glutathione
- DAB:
-
Diaminobenzidine
- DHA:
-
Dehydroascorbate
- DHAR:
-
Dehydroascorbate reductase
- Fer:
-
Ferritin
- GPX:
-
Glutathione peroxidase
- GR:
-
Glutathione reductase
- GSSG:
-
Glutathione oxidized
- MDA:
-
Malondialdehyde
- MDHA:
-
Monodehydroascorbate
- MDHAR:
-
Monodehydroascorbate reductase
- NBT:
-
Nitrotetrazolium blue chloride
- Pn:
-
Net photosynthesis rate
- POD:
-
Peroxidase
- Pro:
-
Proline
- PVP:
-
Polyvinyl pyrrolidone
- REC:
-
Relative electronic conductivity
- ROS:
-
Reactive oxygen species
- RWC:
-
Relative water content
- SOD:
-
Superoxide dismutase
References
Abedi T, Pakniyat H (2010) Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J Genet Plant Breed 46:27–34
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Barrs H, Weatherley P (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci 15:413–428
Bates L, Waldren R, Teare I (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Benjamin A, Franoise LC, Marie-Franoise N et al (2012) Nitrogen availability impacts oilseed rape (Brassica napus L.) plant water status and proline production efficiency under water-limited conditions. Planta 236:659–676
Brown J, Brown AP, Davis JB et al (1997) Intergeneric hybridization between Sinapis alba and Brassica napus. Euphytica 93:163–168
Chai L (2011) Ascorbate peroxidase gene from Brassica napus enhances salt and drought tolerances in Arabidopsis thaliana. Afr J Biotechnol 10:18085–18091
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought from genes to the whole plant. Funct Plant Biol 30:239–264
Chaves M, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot-London 103:551–560
Cruz de Carvalho MH (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav 3:156–165
Dionisio Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Fang Y, Xiong L (2015) General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72:673–689
Farooq M, Wahid A, Kobayashi N et al (2009) Plant drought stress: effects, mechanisms and management. J Sustain Agr 29:153–188
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Giannopolitis CN, Ries SK (1977) Superoxide dismutases I. Occurrence in higher plants. Plant Physiol 59:309–314
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gomes FP, Oliva MA, Mielke MS et al (2010) Osmotic adjustment, proline accumulation and cell membrane stability in leaves of Cocos nucifera submitted to drought stress. Sci Hortic 126:379–384
Han Q, Kakubari Y (1996) Drought-dependent responses of photosynthesis, transpiration and water use efficiency of Japanese cypress and Japanese red pine seedlings. J For Res 1:73–78
Hasanuzzaman M, Hossain MA, Da Silva JAT et al (2012) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. Crop stress and its management: perspectives and strategies. Springer, Berlin, pp 261–315
Hodges DM, DeLong JM, Forney CF et al (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hou X, Duan C, Liu G et al (2006) Photosynthesis characters of tree peony in response to soil water content. Acta Agr Boreali-Sin 21:91–94
Hussain M, Mumtaz S (2014) Climate change and managing water crisis: Pakistan’s perspective. Rev Environ Health 29:71–77
Kishor PK, Sangam S, Amrutha R et al (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Li A, Jiang J, Zhang Y et al (2012) Molecular and cytological characterization of introgression lines with yellow seed derived from somatic hybrids between Brassica napus and Sinapis alba. Mol Breed 29:209–219
Li Z, Shi P, Peng Y (2013) Improved drought tolerance through drought preconditioning associated with changes in antioxidant enzyme activities, gene expression and osmoregulatory solutes accumulation in white clover (Trifolium repens L.). Plant Omics 6:481–489
Lisenbee CS, Lingard MJ, Trelease RN (2005) Arabidopsis peroxisomes possess functionally redundant membrane and matrix isoforms of monodehydroascorbate reductase. Plant J 43:900–914
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940
Martínez JP, Silva H, Ledent JF et al (2007) Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur J Agron 26:30–38
Miao Y, Lv D, Wang P et al (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18:2749–2766
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133:481–489
Mittler R, Vanderauwera S, Suzuki N et al (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309
Munné Bosch S, Queval G, Foyer CH (2013) The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol 161:5–19
Pavlović I, Ludwig-Müller J, Salopek-Sondi B (2014) Hormonal profile and antioxidant defense system of Brassica rapa plants during drought and recovery period. Plant Biol 6:21–26
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Prashanth SR, Sadhasivam V, Parida A (2008) Over expression of cytosolic copper/zinc superoxide dismutase from a mangrove plant Avicennia marina in indica Rice var. Pusa Basmati-1 confers abiotic stress tolerance. Transgenic Res 17:281–291
Premachandra G, Saneoka H, Ogata S (1990) Cell membrane stability, an indicator of drought tolerance, as affected by applied nitrogen in soyabean. J Agr Sci 115:63–66
Pyngrope S, Bhoomika K, Dubey R (2013) Reactive oxygen species, ascorbate-glutathione pool, and enzymes of their metabolism in drought-sensitive and tolerant indica rice (Oryza sativa L.) seedlings subjected to progressing levels of water deficit. Protoplasma 250:585–600
Queval G, Issakidis Bourguet E, Hoeberichts FA et al (2007) Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J 52:640–657
Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136
Reddy AR, Chaitanya KV, Vivekanandan M (2004) Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol 161:1189–1202
Saruhan N, Terzi R, Saglam A et al (2009) The relationship between leaf rolling and ascorbate-glutathione cycle enzymes in apoplastic and symplastic areas of Ctenanthe setosa subjected to drought stress. Biol Res 42:315–326
Sharma P, Dubey RS (2005) Drought induces oxidative stress and enhances the activities of antioxidant enzymes in growing rice seedlings. Plant Growth Regul 46:209–221
Shukla N, Awasthi R, Rawat L et al (2012) Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol Biochem 54:78–88
Simon-Sarkadi L, Kocsy G, Várhegyi Á et al (2006) Stress-induced changes in the free amino acid composition in transgenic soybean plants having increased proline content. Biol Plant 50:793–796
Smirnoff N (1993) Tansley review no. 52. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Tang L, Yang XL (2008) Improving potato plants oxidative stress and salt tolerance by gene transfer both of Cu/Zn superoxide dismutase and ascorbate peroxidase. Chin Biotechnol 28:25–31
Tian F, Gong J, Zhang J et al (2013) Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. J Exp Bot 64:1509–1520
Tohidi-Moghadam H, Shirani Rad A, Nour Mohammadi G et al (2009) Effect of super absorbent application on antioxidant enzyme activities in Canola (Brassica napus L.) cultivars under water stress conditions. Am J Agric Biol Sci 4:215–223
Tsugane K, Niwa Y, Ohba YK et al (1999) A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 11:1195–1206
Türkan İ, Bor M, Özdemir F et al (2005) Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci 168:223–231
Wang Q (2006) Effects of drought stress on protective enzymes activities and membrane lipid peroxidation in leaves of soybean seedlings. J Agro-Environ Sci 25:918–921
Wang Y, Soontag K, Rudloff E et al (2005) Intergeneric somatic hybridization between Brassica napus L. and Sinapis alba L. J Integr Plant Biol 47:84–91
Wu N, Guan Y, Shi Y (2011) Effect of water stress on physiological traits and yield in rice backcross lines after anthesis. Energy Proced 5:255–260
Xu L, Lin Z, Tao Q et al (2014) Multiple nuclear factor Y transcription factors respond to abiotic stress in Brassica napus L. PLoS One 9:e111354
Yan L, Zhao X, Xia X et al (2008) Effects of oligochitosan on photosynthetic parameter of Brassica napus seedlings under drought stress. Acta Agron Sin 34:326–329
Yang GP, Rhodes D, Joly RJ (1996) Effects of high temperature on membrane stability and chlorophyll fluorescence in glycinebetaine-deficient and glycinebetaine-containing maize lines. Funct Plant Biol 23:437–443
Zhang D, Yang H, Li X et al (2014) Overexpression of Tamarix albiflonum TaMnSOD increases drought tolerance in transgenic cotton. Mol Breed 34:1–11
Zhao Z, Cai Y, Fu M et al (2008) Response of the soils of different land use types to drought: eco-physiological characteristics of plants grown on the soils by pot experiment. Ecol Eng 34:215–222
Zhao L, Wang W, Song Y (2010) Changes of photosynthesis and membrane damage of Brassica napus L. under soil water stress. J Henan Agr Sci 8:33–35
Zhou S, Hu W, Deng X et al (2012) Overexpression of the wheat aquaporin gene, TaAQP7, enhances drought tolerance in transgenic tobacco. PLoS One 7:e52439
Zhou S, Sun X, Yin S et al (2014) The role of the F-box gene TaFBA1 from wheat (Triticum aestivum L.) in drought tolerance. Plant Physiol Biochem 84:213–223
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grants 31330057, 31501335), the Jiangsu Province Science Foundation (Grants BK20150441, 15KJB180019), China Postdoctoral Science Foundation (2015M570482), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Yangzhou University for Excellent Talent Support Program.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by W Zhou.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xia, L., Yang, L., Sun, N. et al. Physiological and antioxidant enzyme gene expression analysis reveals the improved tolerance to drought stress of the somatic hybrid offspring of Brassica napus and Sinapis alba at vegetative stage. Acta Physiol Plant 38, 88 (2016). https://doi.org/10.1007/s11738-016-2111-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2111-0