Abstract
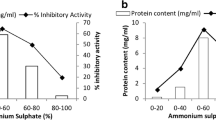
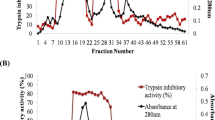
In the present study, trypsin inhibitor extracts of ten kidney bean seed (Phaseolus vulgaris) varieties exhibiting trypsin and gut trypsin-like protease inhibitor activity were tested on Helicoverpa armigera and Spodoptera litura. Trypsin inhibitor protein was isolated and purified using multi-step strategy with a recovery of ~15 % and purification fold by ~39.4. SDS-PAGE revealed a single band corresponding to molecular mass of ~15 kDa and inhibitory activity was confirmed by reverse zymogram analyses. The inhibitor retained its inhibitory activity over a broad range of pH (3–11), temperature (40–60 °C) and thermostability was promoted by casein, CaCl2, BSA and sucrose. The purified inhibitor inhibited bovine trypsin in 1:1 molar ratio. Kinetic studies showed that the protein is a competitive inhibitor with an equilibrium dissociation constant of 1.85 μM. The purified trypsin inhibitor protein was further incorporated in the artificial diet and fed to second instar larvae. A maximum of 91.7 % inhibition was obtained in H. armigera, while it was moderate in S. litura (29 %) with slight varietal differences. The insect bioassay showed 40 and 22 % decrease in larval growth followed by 3 and 2 days delay in pupation of H. armigera and S. litura, respectively. Some of the adults emerged were deformed and not fully formed. Trypsin inhibitor protein was more effective against H. armigera as it showed 46.7 % mortality during larval growth period compared to S. litura (13.3 %).









Similar content being viewed by others
References
Bate NJ, Rothstein SJ (1998) C6-volatiles derived from the lipoxygenase pathway induce a subset of defense-related genes. Plant J 16(5):561–569
Benjakul S, Visessanguan W, Thummaratwasik P (2000) Isolation and characterization of trypsin inhibitors from some Thai legume seeds. J Food Biochem 24:107–127
Bhattacharyya A, Mazumdar S, Leighton SM, Babu CR (2006) A Kunitz proteinase inhibitor from Archidendron ellipticum seeds: purification, characterization and kinetic properties. Phytochem 67:232–241
Bodhe AM (1991) Purification and properties of a subtilisin inhibitor and associated trypsin inhibitor from Dolichos biflorus. Biochim Biophys Acta 1073:11–17
Bown DP, Wilkinson HS, Gatehouse JA (1998) Midgut carboxypeptidase from Helicoverpa armigera (Lepidoptera: Noctuidae) larvae: enzyme characterization, cDNA cloning and expression. Insect Biochem Mol Biol 28:739–749
Cheftel JC, Cuq JL, Lorient D (1985) Amino acids, peptides, and proteins. In: Fennema OR (ed) Food chemicals. Marcel Dekker, New York, pp 245–369
Davis BJ (1964) Disc electrophoresis II: methods and application to human serum. Ann NY Acad Sci 121:404–429
Finney DJ (ed) (1952) Probit analysis. Cambridge University Press, Cambridge
Franco-Fraguas L, Pla A, Ferreira F, Massaldi H, Suarez N, Batista-Viera F (2003) Preparative purification of soybean agglutinin by affinity chromatography and its immobilization for polysaccharide isolation. J Chromatogr B 90(1–2):365
Garcia-Carreno FL, Dimes LE, Haard NF (1993) Substrate–gel electrophoresis for composition and molecular weight of proteinase or proteinaceous proteinase inhibitors. Anal Biochem 214:65–69
Giri AP, Chougule NP, Telang MA, Gupta VS (2005) Engineering insect tolerant plants using plant defensive proteinase inhibitors. In: Pandalai SG (ed) Recent research developments in phytochemistry, 8. Research Signpost, India, pp 117–137
Godbole SA, Krishna TG, Bhatia CR (1994) Purification and characterization of protease inhibitors from pigeon pea (Cajanus cajan (L) Millsp) seeds. J Sci Food Agric 64:87–93
Graber M, Condoret JS (1992) Preparative anion-exchange chromatography of soybean trypsin inhibitor: the alternative of column-overload methods. J Chromatogr 584:115
Graham JS, Ryan CA (1997) Accumulation of metallo-carboxy peptidase inhibitor in leaves of wounded potato plants. Biochem Biophys Res Commun 101:1164–1170
Gruden K, Kuipers AGJ, Guncar G, Slapar N, Strukelj B, Jongsma MA (2004) Molecular basis of Colorado potato beetles adaptation to potato plant defense at the level of digestive cysteine proteinases. Insect Biochem Mol Biol 34:365–375
Gupta P, Dhawan K, Malhotra SP, Singh R (2000) Purification and characterization of trypsin inhibitor from seeds of faba bean (Vicia faba L.). Acta Physiol Plant 22:433–438
Hajela N, Pande AH, Sharma S, Rao DN, Hajela K (1999) Studies on a double headed protease inhibitor from Phaseolus mungo. J Plant Biochem Biotechnol 8:57–60
Harsulkar AM, Giri AP, Patankar AG, Gupta VS, Sainani MN, Ranjekar PK, Deshpande VV (1999) Successive use of non-host plant proteinase inhibitors required for effective inhibition of gut proteinases and larval growth of H. armigera. Plant Physiol 121:497–506
Howe RW (1971) A parameter for expressing the suitability of an environment for insect development. J Stored Prod Res 7:63–65
Jouanin L, Bonade-Bottino M, Girard C, Morrot G, Giband M (1998) Transgenic plants for insect resistance. Plant Sci 131:1–11
Kalia V, Chaudhari S, Gujar GT (2001) Optimization of production of nucleopolyhedrovirus of H. armigera throughout larval stages. Phytoparas 29:23–28
Kansal R, Kuhar K, Gupta RN, Gupta VK, Koundal KR (2008a) Screening of indigenous legumes for trypsin inhibitor protein activity. Indian J Agric Biochem 21:54–56
Kansal R, Kumar M, Kuhar K, Gupta RN, Subrahmanyam B, Koundal KR, Gupta VK (2008b) Purification and characterization of trypsin inhibitor from Cicer arietinum L. and its efficacy against H. armigera. Brazilian J Plant Physiol 20:313–322
Klomklao S, Benjakul S, Kishimura H, Osako K, Tanaka M (2010a) A heatstable trypsin inhibitor in adzuki bean (Vigna angularis): effect of extraction media, purification and biochemical characteristics. Int J Food Sci Technol 45:163–169
Klomklao S, Benjakul S, Kishimura H, Osako K, Tanaka M (2010b) Effect of salts and polyethylene glycols on the partitioning and recovery of trypsin from hybrid catfish viscera in aqueous two-phase systems. J Food Biochem 34:730–747
Kuhar K, Kansal R, Subrahmanyam B, Koundal KR, Miglani K, Gupta VK (2013) A Bowman–Birk protease inhibitor with anti-feedant and antifungal activity from Dolichos biflorus. Acta Physiol Plant 35:1887–1903
Kumar M, Kumar M, Kansal R, Srivastava PS, Koundal KR (2007) Effect of indigenous legumes protease inhibitor proteins on H. armigera. Prog Agric 7:56–59
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Laskowski M, Qasim MA (2000) What can the structures of enzyme inhibitor complexes tell us about the structures of enzyme substrate complexes? Biochim Biophys Acta 1477:324–337
Liener IE, Kakade ML (1969) Protease inhibitors. In: Liener IE (ed) Toxic constituents of plant foodstuffs. Academic Press, London, pp 14–25
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Macedo MLR, Matos DGG, Machado OLT, Marangoni S, Novello JC (2000) Trypsin inhibitor from Dimorphondra mollis seeds: purification and properties. Phytochem 54:553–558
Macedo MLR, Freire MGM, Cabrini EC, Toyama MH, Novello JC, Marangoni S (2003) A trypsin inhibitor from Peltophorum dubium seeds active against pest proteases and its effect on the survival of Anagasta kuehniella (Lepidoptera: Pyralidae). Biochim Biophys Acta 1621:170–182
Macedo ML, de Sa CM, Freire MD, Parra JR (2004) A Kunitz type trypsin inhibitor of coleopteran proteases, isolated from Adenanthera pavonina L. seeds and its effect on Callosobruchus maculatus. J Agric Food Chem 52:2533–2540
Maggo S, Malhotra SP, Dhawan K, Singh R (1999) Purification and characterization of protease inhibitor from rice bean (Vigna umbellata). J Plant Biochem Biotech 8:61–64
Manjunath TM, Bhatnagar VS, Pawar CS, Sithanantham S (1989) Economic importance of Heliothis spp. in India and assessment of their natural enemies and host plants. In: King EG, Jackson RD (eds) Proceedings of the workshop on biological control of Heliothis: increasing the effectiveness of natural enemies. Far Eastern Regional Office, US Department of Agriculture, New Delhi, pp 197–228
McNiven MA, Grimmelt B, Macleod JA, Voldens H (1992) Biochemical characterization of a low trypsin inhibitor soybean. J Food Sci 57:1375
Nagarkatti S, Prakash A (1974) Rearing of Heliothis armigera (Hubner) on artificial diet. Tech Bull Commonw Inst Biol Cont 17:169–173
Novillo C, Castanera P, Ortego F (1997) Inhibition of digestive trypsin-like proteases from larvae of several lepidopteran species by the diagnostic cysteine proteinase inhibitor E-64. Insect Biochem Mol Biol 27:247–254
Ogiso T, Noda T, Sako Y, Kato Y, Aoyama M (1975) Studies on trypsin inhibitor in barley. I. Purification and some properties. J Biochem 78:9–17
Ohtsubo K (1989) Effects of salts, protease digestions, chemical modifications and heat treatment on the trypsin inhibitory activity of protease inhibitor from Job’s tears (Coix lacryma-jobi L. var. Ma-yuen Stapf). Agric Biol Chem 53:333–339
Oliveira AS, Migliolo L, Aquino RO, Ribeiro JKC, Macedo LLP, Andrade LBS, Bemquerer MP, Santos EA, Kiyota S, Sales MP (2007) Identification of a Kunitz-type proteinase inhibitor from Pithecellobium dumosum seeds with insecticidal properties and double activity. J Agric Food Chem 55:7342–7349
Patankar AG, Harsulkar AM, Giri AP, Gupta VS, Sainani MN, Ranjekar PK, Deshpande VV (1999) Diversity in inhibitors of trypsin and H. armigera gut proteinases in chickpea (Cicer arietinum) and its wild relatives. Theor Appl Genet 199:719–726
Patankar AG, Giri AP, Harsulkar AM, Sainani MN, Deshpande VV, Ranjekar PK, Gupta VS (2001) Complexity in specificities and expression of H. armigera gut proteases explains polyphagous nature of the insect pest. Insect Biochem Mol Biol 31:453–464
Ryan CA (1973) Proteolytic enzymes and their inhibitors in plants. Annu Rev Plant Physiol 24:173–196
Ryan CA (1990) Proteinase inhibitors in plants, genes for improving defenses against insects and pathogens. Annu Rev Phytopathol 28:425–449
Scarpi D, McBride JD, Leatherbarrow RJ (2004) Inhibition of human beta-tryptase by Bowman–Birk inhibitor derived peptides: creation of a new tri-functional inhibitor. Bioorg Med Chem 12(23):6045
Shukla S, Arora R, Sharma HC (2005) Biological activity of soybean trypsin inhibitor and plant lectins against cotton bollworm/legume pod borer, H. armigera. Plant Biotechnol 22:1–6
Srinivasan A, Giri AP, Harsulkar AM, Gatehouse JA, Gupta VS (2005) A Kunitz trypsin inhibitor from chickpea (Cicer arietinum L.) that exerts anti-metabolic effect on podborer (H. armigera) larvae. Plant Mol Biol 57:359–374
Sudheendra K, Mulimani VH (2002) Effect of feeding legume proteinase inhibitors on H. armigera gut proteinase activity. Intern Chickpea Pigeonpea Newslett 9:51–53
Tamayo MC, Rufat M, Bravo JM, San Segundo B (2000) Accumulation of a maize proteinase inhibitor in response to wounding and insect feeding, and characterization of its activity towards digestive proteinase of Spodoptera littoralis larvae. Planta 211:62–71
Tatyana AV, Tatyana AR, Galina VK, Vladimr VM (1998) Kunitz type protease inhibitors from intact and Phytophthora infected potato tubers. FEBS Lett 426:131–134
Telang M, Srinivasan A, Patankar A, Harsulkar A, Joshi V, Damle A, Deshpande V, Sainani M, Ranjekar P, Gupta G, Birah A, Rani S, Kachole M, Giri A, Gupta V (2003) Bitter gourd proteinase inhibitors: potential growth inhibitors of H. armigera and Spodoptera litura. Phytochem 63:643–652
Tetenbaum J, Miller LM (2001) A new spectroscopic approach to examining the role of disulphide bonds in the structure and unfolding of soybean trypsin inhibitor. Biochem 40:12215–12219
Vacconcelos IM, Siebra EA, Maia AAB, Moreira RA, Neto AF, Campelo GJA (1997) Composition, toxic and anti-nutritional factors of newly developed cultivars of Brazilian soybean (Glycine max). J Sci Food Agric 75:419–426
Zhao Y, Botella MA, Subramanian L, Niu XM, Nielsen SS, Bressan RA, Hasegawa PM (1996) Two wound-inducible soybean cysteine proteinase inhibitors have greater insect digestive proteinase inhibitory activities than a constitutive homolog. Plant Physiol 111:1299–1306
Acknowledgments
Ms. Anuradha Mittal greatly acknowledges the financial assistance from Department of Science and Technology, India as INSPIRE Junior Research Fellowship under INSPIRE Fellowship Scheme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Barna.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mittal, A., Kansal, R., Kalia, V. et al. A kidney bean trypsin inhibitor with an insecticidal potential against Helicoverpa armigera and Spodoptera litura . Acta Physiol Plant 36, 525–539 (2014). https://doi.org/10.1007/s11738-013-1433-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1433-4