Abstract
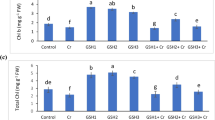
Perennial pepperweed (Lepidium latifolium Linn.) is a preferred ‘phytofood’ that is available for the longest period of a year in Ladakh. Present study was undertaken to identify the mechanism of redox homeostasis and understand factors responsible for its biochemical superiority during low temperatures. Results reveal that despite the stressful environment at higher altitude, the cellular conditions are more reducing for this plant. The reducing environment is maintained by significant induction of GSH rather than changes in its oxidation state, which changes the redox potential by 12 mV. Lower ratio of NADP+/NADPH and induction of new antioxidative isozymes at Leh (3,505 m) suggest crucial role of redox regulation in adaptation. These new proteins have higher thiol content and could provide an efficient redox sensing mechanism in Lepidium latifolium that respond through GSH/NADPH redox buffers. In vitro feeding experiment suggested that GSH plays an important role in induction of antioxidant enzymes, which may not be the direct consequence of H2O2 accumulation. It needs to be further investigated whether its responsive redox metabolism has some role in its invasive growth in riparian plains of America.





Similar content being viewed by others
Abbreviations
- ASC/DHA:
-
Reduced and oxidized form of ascorbate
- DCPIP:
-
2,6-dichlorophenol-indophenol
- DPPH:
-
1,1-diphenyl-2-picrylhydrazyl
- GSH/GSSG:
-
Reduced and oxidized form of glutathione
- MDA:
-
Malondialdehyde
- NAD+/NADH:
-
Reduced and oxidized form of nicotinamide adenine dinucleotide
- NADP+/NADPH:
-
Reduced and oxidized form of nicotinamide adenine dinucleotide phosphate
- ROS:
-
Reactive oxygen species
- TBA:
-
2-thiobarbituric acid
References
Abrol E, Vyas D, Koul S (2012) Metabolic shift from secondary metabolite production to induction of anti-oxidative enzymes during NaCl stress in Swertia chirata Buch-Ham. Acta Physiol Plant 34:541–546
Anderson MD, Prasad TK, Stewart CR (1995) Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol 109:1247–1257
Aravind P, Prasad MN (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate-glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43:107–116
Aslam M, Sinha VB, Singh RK, Anandhan S, Ahmed Z, Pande V (2010) Isolation of cold stress-responsive genes from Lepidium latifolium by suppressive subtraction hybridization. Acta Physiol Plant 32:205–210
Bartoli CG, Tambussi EA, Diego F, Foyer CH (2009) Control of ascorbic acid synthesis and accumulation and glutathione by the incident light red/far red ratio in Phaseolus vulgaris leaves. FEBS Lett 583:118–122
Buchanan BB, Balmer Y (2005) Redox regulation: a broadening horizon. Ann Rev Plant Bio 56:187–220
Chen H, Qualls RG, Miller GC (2002) Adaptive responses of Lepidium latifolium to soil flooding: biomass allocation, adventitious rooting, aerenchyma formation and ethylene production. Environ Exp Bot 48:119–128
Chronopoulou E, Madesis P, Asimakopoulou B, Dimitrios P, Tsaftaris A, Labrou NE (2012) Catalytic and structural diversity of the fluazifop-inducible glutathione transferases from Phaseolus vulgaris. Planta 235(6):1253–1269
Colville L, Smirnoff N (2008) Antioxidant status, peroxidase activity, and PR protein transcript levels in ascorbate-deficient Arabidopsis thaliana vtc mutants. J Exp Bot 59:3857–3868
Eskling M, Arvidsson PO, Akerlund H-K (1997) The xanthophyll cycle, its regulation and components. Physiol Plant 100:806–816
Fendt SM, Buescher JM, Rudroff F, Picotti P, Zamboni N, Sauer U (2010) Trade off between enzyme and metabolite efficiency maintains metabolic homeostasis upon perturbations in enzyme capacity. Mol Sys Biol 6:356
Foyer CH, Noctor G (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11:861–905
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Golding AJ, Finazzi G, Johnson GN (2004) Reduction of the thylakoid electron transport chain by stromal reductants: evidence for activation of cyclic electron transport upon dark adaptation or under drought. Planta 220:356–363
Gomez LD, Noctor G, Knight M, Foyer CH (2004) Regulation of calcium signaling and gene expression by glutathione. J Exp Bot 55:1851–1859
Grace SC, Logan BA (1996) Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol 112:1631–1640
Guleria S, Tiku AK, Singh G, Vyas D, Bhardwaj A (2011) Antioxidant activity and protective effect against plasmid DNA strand scission of leaf, bark, and Heartwood Extracts from Acacia catechu. J Food Sci 76:959–964
Hancock JT, Desikan R, Neill SJ, Cross AR (2004) New equations for redox and nano-signal transduction. J Theor Biol 226(1):65–68
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 12:189–198
Hultén E, Fries M (eds) (1986) Atlas of North European vascular plants, part I-III, maps and commentaries. Koeltz Scientific books, Germany
Jubany-Mari T, Alegre-Batlle L, Jiang K, Feldman LJ (2010) Use of a redox-sensitive GFP (c-roGFP1) for real-time monitoring of cytosol redox status in Arabidopsis thaliana water-stressed plants. FEBS Lett 584:889–897
Karpinski S, Escobar C, Karpinska B, Creissen G, Mullineaux PM (1997) Photosynthetic electron transport regulate the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9:627–640
Kornas A, Kuźniak E, Slesak I, Miszalski Z (2010) The key role of the redox status in regulation of metabolism in photosynthesizing organisms. Acta Biochem Pol 57:143–151
Kramer DM, Evans JR (2011) The importance of energy balance in improving photosynthetic productivity. Plant Physiol 155:70–78
Kranner I, Birtić S, Anderson KM, Pritchard HW (2006) Glutathione half-cell reduction potential: a universal stress marker and modulator of programmed cell death? Free Rad Biol Med 40:2155–2165
Levitt J (1962) A sulphydryl-disulfide hypothesis of frost injury and resistance in plants. J Theor Biol 3:355–391
Lowry OH, Rosebrough NJ, Farr AL, Randall RL (1951) Protein measurement with the folin-phenol reagent. J Biol Chem 193:265–275
Mehler AH (1951) Studies on reactions of illuminated chloroplasts I mechanisms of the reduction of oxygen and other hill reagents. Arch Biochem Biophys 33:65–77
Mhamdi A, Hager J, Chaouch S, Queval G, Han Y, Taconnat L, Saindrenan P, Gouia H, Isaakidis-Bourguet E, Renou J-P, Noctor G (2010) Arabidopsis glutathione reductase I plays a crucial role in leaf responses to intracellular hydrogen peroxide and in ensuring appropriate gene expression through both salicylic and jasmonic acid signalling pathways. Plant Physiol 153(1144):1160
Muller P, Li XP, Niyogi KK (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125:1558–1566
Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429:579–582
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Navarro E, Alonso J, Rodriguez R, Trujillo J, Boada J (1994) Diuretic action of an aqueous extract of Lepidium latifolium L. J Ethnopharmacol 41:65–69
Noctor G (2006) Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant Cell Environ 29:409–425
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol Plant Mol Biol 49:249–279
Noctor G, Mhamdi A, Chaouchi S, Han Y, Neukermans J, Marquez–Garcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Pal Murugan M, Raj J, Kumar PG, Gupta S, Singh SB (2010) Phytofoods of Nubra valley, Ladakh-The cold desert. Indian J Trad Knowl 9(2):303–308
Pimentel D, Lach L, Zuniga R, Morrison D (2000) Environmental and economic costs of non indigenous species in the United States. BioScience 50:53–65
Queval G, Noctor G (2007) A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: application to redox profiling during Arabidopsis rosette development. Anal Biochem 363:58–69
Rajagopal S, Bukhov NG, Tajmir-Riahi H, Carpentier R (2003) Control of energy dissipation and photochemical activity in photosystem I by NADP-dependent reversible conformational changes. Biochemistry 42:11839–11845
Rao MV, Hale BA, Ormrod DP (1995) Amelioration of Ozone-lnduced oxidative damage in wheat plants grown under high carbon dioxide role of antioxidant enzymes. Plant Physiol 109:421–432
Rollins RC (1993) The Cruciferae of Continental North America. Stanford University Press, Stanford
Sawhney SK, Singh R (eds) (2009) Introductory practical biochemistry, 2nd edn. Narosa Publishing House, New Delhi
Schafer FQ, Buettner GR (2001) Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Rad Biol Med 30(11):1191–1212
Scheibe R, Backhausen JE, Emmerlich V, Simone H (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56:1481–1489
Schultze-Motel W (ed) ((1986)) Lepidium latifolium. Illustrierte Flora von Mittel-europa, 3rd edn. Verlag Paul Parey, Berlin
Smirnoff N (2000) Ascorbate biosynthesis and function in photoprotection. Royal Soc 355:1455–1464
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5, 50-dithiobis(2nitrobenzoic acid). Anal Biochem 175:408–413
Snedecor GW, Cochran WG (1989) Statistical methods, 8th edn. Iowa State University Press, Ames
Sonnentag O, Detto M, Runkle BRK, Teh YA, Silver WL, Kelly M, Baldocchi DD (2011) Carbon dioxide exchange of a pepperweed (Lepidium latifolium L.) infestation: How do flowering and mowing affect canopy photosynthesis and autotrophic respiration? J Geophys Res 116:G01021. doi:10.1029/2010JG001522
Tabassum N, Ahmad F (2011) Role of natural herbs in the treatment of hypertension. Pharmacogn 9:30–34
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27:502–522
Vyas D, Kumar S (2005a) Purification and partial characterization of a low temperature responsive Mn-SOD from tea (Camellia sinensis(L.) O. Kuntze.). Biochem Biophys Res Comm 329:831–838
Vyas D, Kumar S (2005b) Tea (Camellia sinensis (L.) O. Kuntze) clone with lower period of winter dormancy exhibits lesser cellular damage in response to low temperature. Plant Physio Biochem 43:383–388
Woodbury W, Spencer AK, Stahman MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44:301–305
Yabuta Y, Mieda T, Rapolu M, Nakamura A, Motoki T, Maruta T, Yoshimura K, Ishikawa T, Shigeoka S (2007) Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J Exp Bot 58:2661–2671
Young JA, Turner CE, James LF (1995) Perennial pepperweed. Rangelands 17:121–123
Acknowledgments
Authors would like to thank anonymous reviewers for suggesting necessary changes in improving the quality of manuscript. Authors thank the Director, IIIM, Jammu for providing necessary facilities to carry out the work. Authors are grateful to the Council of Scientific and Industrial Research (CSIR), Government of India, for financial support under CSIR- networking project (BSC-0109) on ‘Plant Diversity: Studying adaptation biology and understanding/exploiting medicinally important plants for useful bioactives (SIMPLE)’.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Bavaresco.
Rights and permissions
About this article
Cite this article
Kaur, T., Bhat, H.A., Raina, A. et al. Glutathione regulates enzymatic antioxidant defence with differential thiol content in perennial pepperweed and helps adapting to extreme environment. Acta Physiol Plant 35, 2501–2511 (2013). https://doi.org/10.1007/s11738-013-1286-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1286-x