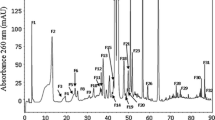
Abstract
The antidiabetic properties of Cecropia obtusifolia are attributed to chlorogenic acid (CGA) and isoorientin (ISO) phenolic compounds; both compounds possess hypoglycemic, hypolipidemic, and antioxidant properties. As a potential strategy for an adequate supply of authentic plant raw material, the aim of this study was to establish in vitro conditions for the development of cell suspension cultures that produce these bioactive compounds. Callus cultures of leaf explants from acclimatized tree and in vitro plantlets were set up using different auxin levels; treatments with 2,4-dichlorophenoxyacetic acid (2,4-D) and α-naphthalene acetic acid (NAA) to 8.92 µM with 6-benzylaminopurine (BAP) at 2.22 μM stimulate highest callus production. Seedling cotyledon, hypocotyl, leaf, and stem explants developed calli bearing roots with 2,4-D. With NAA, hypocotyl, cotyledon, and leaf explants developed morphogenic calli; 75% of stem explants formed calli, and the remaining calli developed shoots. Determined CGA concentrations in calli were similar to those detected in the leaves of wild trees, and ISO was not produced. Cell suspension cultures were established from leaf explants friable calli with 8.92 μM 2,4-D in combination with 2.22 μM BAP, employing 4 and 5% inocula in fresh weight; CGA levels were maintained and ISO was produced only at the end of logarithmic growth. On diminishing nitrate content in Murashige and Skoog (MS) medium to 8.0 mM, maximum cell biomasses diminished, CGA production is increased and twice with 16.0 and, instead of CGA production is tripled and quadrupled with 16.0 and 8.0 mM nitrates, respectively, and ISO synthesis was induced earlier and for a longer time period, increasing its levels at the end of culture. Two compounds with ultraviolet spectra similar to those of caffeic and ferulic acids were formed. Our results offer a protocol of cell suspension cultures for C. obtusifolia bioactive production and hypoglycemic property conservation.




Similar content being viewed by others
Abbreviations
- BAP:
-
6-Benzylaminopurine
- CGA:
-
Chlorogenic acid
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- DM:
-
Diabetes mellitus
- DW:
-
Dry weight
- IBA:
-
Indolebutyric acid
- MSD:
-
Minimal significant difference
- IAA:
-
Indole-3-acetic acid
- ISO:
-
Isoorientin
- MS:
-
Murashige and Skoog
- NAA:
-
α-Naphthalene acetic acid
References
Aguilar A, Camacho JR, Chino S, Jácquez P, López ME (1994) Herbario Medicinal del Instituto Mexicano del Seguro Social. Instituto Mexicano del Seguro Social, México, p 142
Aguilar A, Camacho JR, Chino S, Jácquez P, López ME (1998) Plantas Medicinales del Herbario del IMSS, Su Distribución por Enfermedades. Instituto Mexicano del Seguro Social y Grupo Roche Syntex de México, México, p 40
Alonso-Castro AJ, Miranda-Torres AC, González-Chávez MM, Salazar-Olivo LA (2008) Cecropia obtusifolia Bertol and its active compound, chlorogenic acid, stimulate 2-NBD glucose uptake in both insulin-sensitive and insulin-resistant 3T3 adipocytes. J Ethnopharmacol 120:458–464. doi:10.1016/j.ep.2008.09.019
Andrade-Cetto A, Wiedenfeld H (2001) Hypoglycemic effect of Cecropia obtusifolia on streptozotocin diabetic rats. J Ethnopharmacol 78:145–149. doi:10.1016/S0378-8741(01)00335-X
Argueta A, Cano L, Asselein L, Rodarte ME (1994) Atlas de las plantas de la medicina tradicional mexicana, vol II. Instituto Nacional Indigenista (INI), México, pp 706–707
Armstrong GM, Rohrbaugh EL, Rice EL, Wender SH (1970) The effect of nitrogen deficiency on the concentration of caffeoylquinic acids and scopolin in tobacco. Phytochemistry 9:945–948
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Farzami-Shepher M, Ghorbanli M (2002) Effects of nutritional factors on the formation of anthraquinones in callus cultures of Rheum ribes. Plant Cell Tissue Organ Cult 68:171–175. doi:10.1023/A:1013837232047
Fritz C, Palacios N, Fiel R, Stitt M (2006) Regulation of secondary metabolism by the carbon-nitrogen status in tobacco: nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J 46:533–548. doi:10.1111/j.1365-313X.2006.02715.x
Fujita Y, Hara Y, Suga Ch, Morimoto T (1981) Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon. Plant Cell Rep 1(2):61–63. doi:10.1007/BF00269273
Gillet F, Mesnard F, Fliniaux O, Monti JP, Fliniaux M (1999) Chlorogenic acid in a Nicotiana plumbaginifolia cell suspension. Plant Physiol Biochem 37:869–875. doi:10.1016/S0981-9428(99)00111-4
Herrera-Arellano A, Aguilar-Santamaría L, García-Hernández B, Nicasio-Torres P, Tortoriello J (2004) Clinical trial of Cecropia obtusifolia and Marrubium vulgare leaf extracts on blood glucose and serum lipids in type 2 diabetics. Phytomedicine 11:561–566. doi:10.1016/j.phymed.2004.01.006
Ko FN, Chu CC, Lin CN, Chang CC, Teng CM (1998) Isoorientin- 6″-O-glucoside, a water-soluble antioxidant isolated from Gentiana arisanensis. Biochim Biophys Acta 1389:81–90
Kovácik J, Repcak M, Kron I (2006) Nitrogen deficiency induced changes of free amino acids and coumarin contents in the leaves of Matricaria chamomilla. Acta Physiol Plant 28:159–164. doi:10.1007/s11738-006-0042-x
La Pierre LM (2001) Vegetative propagation of Cecropia obtusifolia (Cecropiaceae). Rev Biol Trop 49:973–976
McNally DJ, Wurms KV, Labbé C, Quideau S, Bélanger RR (2003) Complex C-glycosyl flavonoid phytoalexins from Cucumis sativus. J Nat Prod 66:1280–1283. doi:10.1021/np030150y
Mellado V, Lozoya M (1984) Effect of the aqueous extract of Cecropia obtusifolia on the blood sugar of normal and pancreatectomized dogs. Int J Crude Drug Res 22:11–16
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nakagawa K, Fukui H, Tabata M (1986) Hormonal regulation of berberine production in cell suspension cultures of Thalictrum minus. Plant Cell Rep 5(1):69–71. doi:10.1007/BF00269722
Nalawade SM, Tsay HS (2004) In vitro propagation of some important Chinese medicinal plants and their sustainable usage. In Vitro Cell Dev Biol Plant 40:143–454
Namdeo AG (2007) Plant cell elicitation for production of secondary metabolites: a review. Phcog Rev 1(1):69–79
NCSS (2001) NCSS—Number Cruncher Statistical Software. Kaysville, Utah
Nicasio-Torres P, Aguilar-Santamaría L, Aranda E, Ortiz S, González M (2005) Hypoglycemic effect and chlorogenic acid content in two Cecropia species. Phytother Res 19:661–664. doi:10.1002/ptr.1722
Nicasio-Torres P, Erazo-Gómez JC, Cruz-Sosa F (2009) In vitro propagation of two antidiabetic species known as guarumbo: Cecropia obtusifolia and Cecropia peltata. Acta Physiol Plant 31:905–914. doi:10.1007/s11738-009-0304-5
Nigra HM, Alvarez MA, Giulietti AM (1990) Effect of carbon and nitrogen sources on growth and solasodine production in batch suspension cultures of Solanum eleagnifolium Cav. Plant Cell Tissue Organ Cult 21(1):55–60. doi:10.1007/BF00034492
Pérez RM, Ocegueda A, Muñoz JL, Ávila JG, Morrow WW (1984) A study of the hypoglycemic effect of some Mexican plants. J Ethnopharmacol 12:253–262. doi:10.1016/0378-8741(84)90054-0
Poornananda Madhava N, Shirugumbi Hanamanthagouda M, Murthy HosakatteNiranjana (2011) Effects of macro elements and nitrogen source on biomass accumulation and bacoside A production from adventitious shoot cultures of Bacopa monnieri (L.). Acta Physiol Plant 33(4):1553–1557. doi:10.1007/s11738-010-0675-7
Ramachandra-Rao S, Ravishankar G (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153. doi:10.1016/S0734-9750(02)00007-1
Revilla-Monsalve MC, Andrade-Cetto A, Palomino-Garibay MA, Wiedenfeld H, Islas-Andrade S (2007) Hypoglycemic effect of Cecropia obtusifolia Bertol aqueous extract on type 2 diabetic patients. J Ethnopharmacol 111:636–640. doi:10.1016/j.jep.2007.01.014
Rodríguez de Sotillo DV, Hadley M (2002) Chlorogenic acid modifies plasma and liver concentrations of: cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J Nutr Biochem 13:717–726. doi:10.1016/S0955-2863(02)00231-0
Román-Ramos R, Flores-Sáenz JL, Partida-Hernández G, Lara-Lemus A, Alarcón-Aguilar F (1991) Experimental study of the hypoglycemic effect of some antidiabetic plants. Arch Invest Med (Mex) 22:87–93
Rout GR, Samantaray S, Das P (2000) In vitro manipulation and propagation of medicinal plants. Biotechnol Adv 18:91–120. doi:10.1016/S0734-9750(99)00026-9
Sezik E, Aslan M, Yesilada E, Ito S (2005) Hypoglycaemic activity of Gentiana oliveri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci 76:1223–1238. doi:10.1016/j.lfs.2004.07.024
Shudha G, Ravishanka GA (2002) Involvement and interaction of various signaling compounds on the plant metabolic events during defense response, resistance to stress factors, formation of secondary metabolites and their molecular aspects. Plant Cell Tissue Organ Cult 71:181–212. doi:10.1023/A:1020336626361
Stafford AM (2002) Plant cell cultures as a source of bioactive small molecules. Curr Opin Drug Discov Dev 5:296–303
Urbanczyk-Wochniak E, Fernie AR (2004) Metabolic profiling reveals that altered nitrogen nutrient regimes have diverse effects on the metabolism of hydroponically-grown tomato (Solanum lycopersicum) plants. J Exp Bot 56:309–321
Vanisree M, Lee C-Y, Lo S-F, Nalawade SM, Lin C-Y, Tsay H-S (2004) Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot Bull Acad Sin 45:1–22
Wang J, Liao X, Zhang H, Du J, Chen P (2003) Accumulation of chlorogenic acid in cell suspension cultures of Eucommia ulmoides. Plant Cell Tissue Organ Cult 74:193–195
Mora-Izquierdo A, Nicasio-Torres MP, Sepúlveda-Jiménez G, Cruz-Sosa F Changes in biomass allocation and phenolic compounds accumulation due to the effect of light and nitrate supply in Cecropia peltata plants. Acta Physiol Plant (submitted)
Zhong JJ (2001) Biochemical engineering of the production of plant-specific secondary metabolites by cell suspension cultures. Adv Biochem Eng/Biotechnol 72:1–26
Acknowledgments
MP Nicasio-Torres is grateful for the financial support (2004/077) provided for this research by FOFOI-IMSS, and to Prof. Abigail Aguilar of the Medicinal Plant Herbarium of the Mexican Social Security Institute (IMSSM) for plant material certification. Further appreciation is expressed, especially to my friend, Dr. Eduardo Aranda-Escobar† for his invaluable help.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Lojkowska.
Rights and permissions
About this article
Cite this article
Nicasio-Torres, M.P., Meckes-Fischer, M., Aguilar-Santamaría, L. et al. Production of chlorogenic acid and isoorientin hypoglycemic compounds in Cecropia obtusifolia calli and in cell suspension cultures with nitrate deficiency. Acta Physiol Plant 34, 307–316 (2012). https://doi.org/10.1007/s11738-011-0830-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0830-9