Abstract
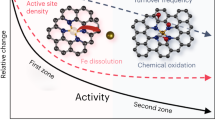
In recent years, Fe-N-C catalyst is particularly attractive due to its high oxygen reduction reaction (ORR) activity and low cost for proton exchange membrane fuel cells (PEMFCs). However, the durability problems still pose challenge to the application of Fe-N-C catalyst. Although considerable work has been done to investigate the degradation mechanisms of Fe-N-C catalyst, most of them are simply focused on the active-site decay, the carbon oxidation, and the demetalation problems. In fact, the 2e− pathway in the ORR process of Fe-N-C catalyst would result in the formation of H2O2, which is proved to be a key degradation source. In this paper, a new insight into the effect of potential on degradation of Fe-N-C catalyst was provided by quantifying the H2O2 intermediate. In this case, stability tests were conducted by the potential-static method in O2 saturated 0.1 mol/L HClO4. During the tests, H2O2 was quantified by rotating ring disk electrode (RRDE). The results show that compared with the loading voltage of 0.4 V, 0.8 V, and 1.0 V, the catalysts being kept at 0.6 V exhibit a highest H2O2 yield. It is found that it is the combined effect of electrochemical oxidation and chemical oxidation (by aggressive radicals like H2O2/radicals) that triggered the highest H2O2 release rate, with the latter as the major cause.
Similar content being viewed by others
References
Martinez U, Komini Babu S, Holby E F, et al. Progress in the development of Fe-based PGM-free electrocatalysts for the oxygen reduction reaction. Advanced Materials, 2019, 31(31): 1806545
Konnerth H, Matsagar B M, Chen S S, et al. Metal-organic framework (MOF)-derived catalysts for fine chemical production. Coordination Chemistry Reviews, 2020, 416: 213319
Yang L J, Shui J L, Du L, et al. Carbon-based metal-free ORR electrocatalysts for fuel cells: past, present, and future. Advanced Materials, 2019, 31(13): 1804799
Banham D, Ye S Y. Current status and future development of catalyst materials and catalyst layers for proton exchange membrane fuel cells: an industrial perspective. ACS Energy Letters, 2017, 2(3): 629–638
Zhang L, Si R, Liu H, et al. Atomic layer deposited Pt-Ru dual-metal dimers and identifying their active sites for hydrogen evolution reaction. Nature Communications, 2019, 10(1): 4936
Liu M L, Zhao Z P, Duan X F, et al. Nanoscale structure design for high-performance Pt-based ORR catalysts. Advanced Materials, 2019, 31(6): 1802234
Chang Q W, Xu Y, Zhu S Q, et al. Pt-Ni nanourchins as electrocatalysts for oxygen reduction reaction. Frontiers in Energy, 2017, 11(3): 254–259
Guo Y, Tang J, Henzie J, et al. Assembly of hollow mesoporous nanoarchitectures composed of ultrafine Mo2C nanoparticles on N-doped carbon nanosheets for efficient electrocatalytic reduction of oxygen. Materials Horizons, 2017, 4(6): 1171–1177
Tan H, Li Y, Kim J, et al. Sub-50 nm iron-nitrogen-doped hollow carbon sphere-encapsulated iron carbide nanoparticles as efficient oxygen reduction catalysts. Advancement of Science, 2018, 5(7): 1800120
Tan H, Tang J, Henzie J, et al. Assembly of hollow carbon nanospheres on graphene nanosheets and creation of iron-nitrogen-doped porous carbon for oxygen reduction. ACS Nano, 2018, 12(6): 5674–5683
Cai J J, Zhou Q Y, Liu B, et al. A sponge-templated sandwich-like cobalt-embedded nitrogen-doped carbon polyhedron/graphene composite as a highly efficient catalyst for Zn-air batteries. Nanoscale, 2020, 12(2): 973–982
Zhang X, Chen A, Zhong M, et al. Metal-organic frameworks (MOFs) and MOF-derived materials for energy storage and conversion. Electrochemical Energy Reviews, 2019, 2(1): 29–104
Chueh C C, Chen C I, Su Y A, et al. Harnessing MOF materials in photovoltaic devices: recent advances, challenges, and perspectives. Journal of Materials Chemistry A, Materials for Energy and Sustainability, 2019, 7(29): 17079–17095
Banham D, Choi J Y, Kishimoto T, et al. Integrating PGM-free catalysts into catalyst layers and proton exchange membrane fuel cell devices. Advanced Materials, 2019, 31(31): 1804846
Doustkhah E, Lin J, Rostamnia S, et al. Development of sulfonic-acid-functionalized mesoporous materials: synthesis and catalytic applications. Chemistry (Weinheim an der Bergstrasse, Germany), 2019, 25(7): 1614–1635
Liao Y T, Nguyen V C, Ishiguro N, et al. Engineering a homogeneous alloy-oxide interface derived from metal-organic frameworks for selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Applied Catalysis B: Environmental, 2020, 270: 118805
Xia W, Tang J, Li J, et al. Defect-rich graphene nanomesh produced by thermal exfoliation of metal-organic frameworks for the oxygen reduction reaction. Angewandte Chemie International Edition, 2019, 58(38): 13354–13359
Jasinski R. New fuel cell cathode catalyst. Nature, 1964, 201(4925): 1212–1213
Jahnke H, Schönborn M, Zimmermann G. Organic dyestuffs as catalysts for fuel cells. In: Schäfer F P, eds. Physical and Chemical Applications of Dyestuffs. Berlin, Heidelberg: Springer, 1976, 61
Lefèvre M, Proietti E, Jaouen F, et al. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science, 2009, 324(5923): 71–74
Yarlagadda V, Carpenter M K, Moylan T E, et al. Boosting fuel cell performance with accessible carbon mesopores. ACS Energy Letters, 2018, 3(3): 618–621
Cheng N, Zhang L, Doyle-Davis K, et al. Single-atom catalysts: from design to application. Electrochemical Energy Reviews, 2019, 2: 539–573
Shao Y Y, Dodelet J P, Wu G, et al. PGM-free cathode catalysts for PEM fuel cells: a mini-review on stability challenges. Advanced Materials, 2019, 31(31): 1807615
Lefevre M, Dodelet J P. Fe-based catalysts for the reduction of oxygen in polymer electrolyte membrane fuel cell conditions: determination of the amount of peroxide released during electroreduction and its influence on the stability of the catalysts. Electrochimica Acta, 2003, 48: 2749–2760
Artyushkova K, Serov A, Rojas-Carbonell S, et al. Chemistry of multitudinous active sites for oxygen reduction reaction in transition metal-nitrogen-carbon electrocatalysts. Journal of Physical Chemistry C, 2015, 119(46): 25917–25928
Muthukrishnan A, Nabae Y, Okajima T, et al. Kinetic approach to investigate the mechanistic pathways of oxygen reduction reaction on Fe-containing N-doped carbon catalysts. ACS Catalysis, 2015, 5 (9): 5194–5202
Zheng W, Wang L, Deng F, et al. Durable and self-hydrating tungsten carbide-based composite polymer electrolyte membrane fuel cells. Nature Communications, 2017, 8(1): 418
Choi C H, Lim H K, Chung M W, et al. The Achilles’ heel of iron-based catalysts during oxygen reduction in an acidic medium. Energy & Environmental Science, 2018, 11(11): 3176–3182
Goellner V, Armel V, Zitolo A, et al. Degradation by hydrogen peroxide of metal-nitrogen-carbon catalysts for oxygen reduction. Journal of the Electrochemical Society, 2015, 162(6): H403–H414
Kumar K, Dubau L, Mermoux M, et al. On the influence of oxygen on the degradation of Fe-N-C catalysts. Angewandte Chemie International Edition, 2020, 59(8): 3235–3243
Kusoglu A, Weber A Z. New insights into perfluorinated sulfonic-acid ionomers. Chemical Reviews, 2017, 117(3): 987–1104
Yang L M, Bai Y Z, Zhang H J, et al. Nitrogen-doped porous carbon derived from Fe-MIL nanocrystals as an electrocatalyst for efficient oxygen reduction. RSC Advances, 2017, 7(36): 22610–22618
Abe H, Hirai Y, Ikeda S, et al. Fe azaphthalocyanine unimolecular layers (Fe AzULs) on carbon nanotubes for realizing highly active oxygen reduction reaction (ORR) catalytic electrodes. NPG Asia Materials, 2019, 11(1): 57
Ma R, Lin G, Zhou Y, et al. A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts. npj Computational Materials, 2019, 5: 78
Wang W, Jia Q Y, Mukerjee S, et al. Recent insights into the oxygen-reduction electrocatalysis of Fe/N/C materials. ACS Catalysis, 2019, 9(11): 10126–10141
Lu Z Y, Chen G X, Siahrostami S, et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nature Catalysis, 2018, 1(2): 156–162
Macauley N, Papadias D D, Fairweather J, et al. Carbon corrosion in PEM fuel cells and the development of accelerated stress tests. Journal of the Electrochemical Society, 2018, 165(6): F3148–F3160
Chen L N, Yu W S, Wang T, et al. Fluorescence detection of hydroxyl radical generated from oxygen reduction on Fe/N/C catalyst. Science China. Chemistry, 2020, 63(2): 198–202
Choi C H, Baldizzone C, Grote J P, et al. Stability of Fe-N-C catalysts in acidic medium studied by operando Spectroscopy. Angewandte Chemie International Edition, 2015, 54(43): 12753–12757
Choi C H, Choi W S, Kasian O, et al. Unraveling the nature of sites active toward hydrogen peroxide reduction in Fe-N-C catalysts. Angewandte Chemie International Edition, 2017, 56(30): 8809–8812
Cai R, Abdellaoui S, Kitt J P, et al. Confocal raman microscopy for the determination of protein and quaternary ammonium ion loadings in biocatalytic membranes for electrochemical energy conversion and storage. Analytical Chemistry, 2017, 89(24): 13290–13298
Li J, Zhang H, Samarakoon W, et al. Thermally driven structure and performance evolution of atomically dispersed FeN4 sites for oxygen reduction. Angewandte Chemie International Edition, 2019, 58(52): 18971–18980
Ren G Y, Gao L L, Teng C, et al. Ancient chemistry ‘Pharaoh’s Snakes’ for efficient Fe-/N-doped carbon electrocatalysts. ACS Applied Materials & Interfaces, 2018, 10(13): 10778–10785
Acknowledgements
The work was supported by the Thirteenth National Key Point Research and Invention Program (No. 2016YFB0101302).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Gao, Y., Hou, M., Qi, M. et al. New insight into effect of potential on degradation of Fe-N-C catalyst for ORR. Front. Energy 15, 421–430 (2021). https://doi.org/10.1007/s11708-021-0727-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11708-021-0727-2