Abstract
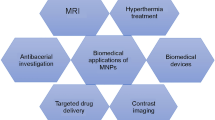
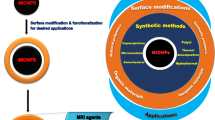
With the rapid improvements in nanomaterials and imaging technology, great progresses have been made in diagnosis and treatment of diseases during the past decades. Fe3O4 magnetic nanoparticles (MNPs) with good biocompatibility and superparamagnetic property are usually used as contrast agent for diagnosis of diseases in magnetic resonance imaging (MRI). Currently, the combination of multiple imaging technologies has been considered as new tendency in diagnosis and treatment of diseases, which could enhance the accuracy and reliability of disease diagnosis and provide new strategies for disease treatment. Therefore, novel contrast agents used for multifunctional imaging are urgently needed. Fe3O4 MNPs are believed to be a potential candidate for construction of multifunctional platform in diagnosis and treatment of diseases. In recent years, there are a plethora of studies concerning the construction of multifunctional platform presented based on Fe3O4 MNPs. In this review, we introduce fabrication methods and modification strategies of Fe3O4 MNPs, expecting great improvements for diagnosis and treatment of diseases in the future.
Similar content being viewed by others
References
Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small, 2008, 4(1): 26–49
Formoso P, Muzzalupo R, Tavano L, et al. Nanotechnology for the environment and medicine. Mini-Reviews in Medicinal Chemistry, 2016, 16(8): 668–675
Hoshyar N, Gray S, Han H B, et al. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine, 2016, 11(6): 673–692
Farokhzad O C, Langer R. Impact of nanotechnology on drug delivery. ACS Nano, 2009, 3(1): 16–20
Baetke S C, Lammers T, Kiessling F. Applications of nanoparticles for diagnosis and therapy of cancer. The British Journal of Radiology, 2015, 88(1054): 20150207
Gupta J. Nanotechnology applications in medicine and dentistry. Journal of Investigative and Clinical Dentistry, 2011, 2(2): 81–88
Zhang A, Lieber C M. Nano-bioelectronics. Chemical Reviews, 2016, 116(1): 215–257
Angle M R, Cui B, Melosh N A. Nanotechnology and neurophysiology. Current Opinion in Neurobiology, 2015, 32: 132–140
Noy A. Bionanoelectronics. Advanced Materials, 2011, 23(7): 807–820
Guerra F D, Attia M F, Whitehead D C, et al. Nanotechnology for environmental remediation: Materials and applications. Molecules, 2018, 23(7): 1760
Hu X, Xu J, Wu C, et al. Ethylenediamine grafted to graphene oxide@Fe3O4 for chromium(VI) decontamination: Performance, modelling, and fractional factorial design. PLoS One, 2017, 12 (10): e0187166
Ma S, Zhan S, Jia Y, et al. Superior antibacterial activity of Fe3O4-TiO2 nanosheets under solar light. ACS Applied Materials & Interfaces, 2015, 7(39): 21875–21883
Sadeghi R, Rodriguez R J, Yao Y, et al. Advances in nanotechnology as they pertain to food and agriculture: Benefits and risks. Annual Review of Food Science and Technology, 2017, 8(1): 467–492
Iavicoli I, Leso V, Beezhold D H, et al. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicology and Applied Pharmacology, 2017, 329: 96–111
Das G, Patra J K, Paramithiotis S, et al. The sustainability challenge of food and environmental nanotechnology: Current status and imminent perceptions. International Journal of Environmental Research and Public Health, 2019, 16(23): 4848
Rossi M, Passeri D, Sinibaldi A, et al. Nanotechnology for food packaging and food quality assessment. Advances in Food and Nutrition Research, 2017, 82: 149–204
Wei M, Le W D. The role of nanomaterials in autophagy. Advances in Experimental Medicine and Biology, 2019, 1206: 273–286
Mohammadinejad R, Moosavi M A, Tavakol S, et al. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy, 2019, 15(1): 4–33
El-Boubbou K. Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine, 2018, 13(8): 929–952
Song C, Sun W, Xiao Y, et al. Ultrasmall iron oxide nanoparticles: Synthesis, surface modification, assembly, and biomedical applications. Drug Discovery Today, 2019, 24(3): 835–844
Gupta A K, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials, 2005, 26(18): 3995–4021
Yuan Y, Ding Z, Qian J, et al. Casp3/7-instructed intracellular aggregation of Fe3O4 nanoparticles enhances T2 MR imaging of tumor apoptosis. Nano Letters, 2016, 16(4): 2686–2691
Chen Y, Zhou Q, Li X, et al. Ultrasmall paramagnetic iron oxide nanoprobe targeting epidermal growth factor receptor for in vivo magnetic resonance imaging of hepatocellular carcinoma. Bioconjugate Chemistry, 2017, 28(11): 2794–2803
Huang J, Wang L, Zhong X, et al. Facile non-hydrothermal synthesis of oligosaccharides coated sub-5 nm magnetic iron oxide nanoparticles with dual MRI contrast enhancement effect. Journal of Materials Chemistry B: Materials for Biology and Medicine, 2014, 2(33): 5344–5351
Martinkova P, Brtnicky M, Kynicky J, et al. Iron oxide nanoparticles: Innovative tool in cancer diagnosis and therapy. Advanced Healthcare Materials, 2018, 7(5): 1700932
Qiao R, Jia Q, Zeng J, et al. Magnetic iron oxide nanoparticles and their applications in magnetic resonance imaging. Acta Biophysica Sinica, 2011, 27(4): 272–288 (in Chinese)
Das R, Rinaldi-Montes N, Alonso J, et al. Boosted hyperthermia therapy by combined ac magnetic and photothermal exposures in Ag/Fe3O4 nanoflowers. ACS Applied Materials & Interfaces, 2016, 8(38): 25162–25169
Nielsen O S, Horsman M, Overgaard J. A future for hyperthermia in cancer treatment? European Journal of Cancer, 2001, 37(13): 1587–1589
Vangijzegem T, Stanicki D, Laurent S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opinion on Drug Delivery, 2019, 16(1): 69–78
Tietze R, Zaloga J, Unterweger H, et al. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochemical and Biophysical Research Communications, 2015, 468(3): 463–470
Hu X, Ma M, Zeng M, et al. Supercritical carbon dioxide anchored Fe3O4 nanoparticles on graphene foam and lithium battery performance. ACS Applied Materials & Interfaces, 2014, 6(24): 22527–22533
Harnchana V, Chaiyachad S, Pimanpang S, et al. Hierarchical Fe3O4-reduced graphene oxide nanocomposite grown on NaCl crystals for triiodide reduction in dye-sensitized solar cells. Scientific Reports, 2019, 9: 1494
Niemiec T, Dudek M, Dziekan N, et al. The method of coating Fe3O4 with carbon nanoparticles to modify biological properties of oxide measured in vitro. Journal of AOAC International, 2017, 100(4): 905–915
Chen S S, Xu H, Xu H J, et al. A facile ultrasonication assisted method for Fe3O4@SiO2-Ag nanospheres with excellent antibacterial activity. Dalton Transactions, 2015, 44(19): 9140–9148
Jiao Z, Zhang Y, Fan H. Ultrasonic-microwave method in preparation of polypyrrole-coated magnetic particles for vitamin D extraction in milk. Journal of Chromatography A, 2016, 1457: 7–13
Montaseri H, Alipour S, Vakilinezhad M A. Development, evaluation and optimization of superparamagnetite nanoparticles prepared by co-precipitation method. Research in Pharmaceutical Sciences, 2017, 12(4): 274–282
Park J, An K, Hwang Y, et al. Ultra-large-scale syntheses of monodisperse nanocrystals. Nature Materials, 2004, 3(12): 891–895
Yu X, Cheng G, Zhou M D, et al. On-demand one-step synthesis of monodisperse functional polymeric microspheres with droplet microfluidics. Langmuir, 2015, 31(13): 3982–3992
Li P, Li L, Zhao Y, et al. Selective binding and magnetic separation of histidine-tagged proteins using Fe3O4/Cu-apatite nanoparticles. Journal of Inorganic Biochemistry, 2016, 156: 49–54
Lu A H, Salabas E L, Schüth F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angewandte Chemie International Edition in English, 2007, 46(8): 1222–1244
Ling D, Lee N, Hyeon T. Chemical synthesis and assembly of uniformly sized iron oxide nanoparticles for medical applications. Accounts of Chemical Research, 2015, 48(5): 1276–1285
Park J, Kadasala N R, Abouelmagd S A, et al. Polymer-iron oxide composite nanoparticles for EPR-independent drug delivery. Biomaterials, 2016, 101: 285–295
Syu W J, Huang C C, Hsiao J K, et al. Co-precipitation synthesis of near-infrared iron oxide nanocrystals on magnetically targeted imaging and photothermal cancer therapy via photoablative protein denature. Nanotheranostics, 2019, 3(3): 236–254
Gan L, Lu Z, Cao D, et al. Effects of cetyltrimethylammonium bromide on the morphology of green synthesized Fe3O4 nanoparticles used to remove phosphate. Materials Science and Engineering C, 2018, 82: 41–45
Wang H, Zhao X, Meng W, et al. Cetyltrimethylammonium bromide-coated Fe3O4 magnetic nanoparticles for analysis of 15 trace polycyclic aromatic hydrocarbons in aquatic environments by ultraperformance, liquid chromatography with fluorescence detection. Analytical Chemistry, 2015, 87(15): 7667–7675
Nosrati H, Salehiabar M, Manjili H K, et al. Preparation of magnetic albumin nanoparticles via a simple and one-pot desolvation and co-precipitation method for medical and pharmaceutical applications. International Journal of Biological Macromolecules, 2018, 108: 909–915
Anbarasu M, Anandan M, Chinnasamy E, et al. Synthesis and characterization of polyethylene glycol (PEG) coated Fe3O4 nanoparticles by chemical co-precipitation method for biomedical applications. Spectrochimica Acta Part A: Molecular Spectroscopy, 2015, 135: 536–539
Li C. A targeted approach to cancer imaging and therapy. Nature Materials, 2014, 13(2): 110–115
LaGrow A P, Besenhard M O, Hodzic A, et al. Unravelling the growth mechanism of the co-precipitation of iron oxide nanoparticles with the aid of synchrotron X-ray diffraction in solution. Nanoscale, 2019, 11(14): 6620–6628
Jun Y W, Huh Y M, Choi J S, et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. Journal of the American Chemical Society, 2005, 127(16): 5732–5733
Hufschmid R, Arami H, Ferguson R M, et al. Synthesis of phasepure and monodisperse iron oxide nanoparticles by thermal decomposition. Nanoscale, 2015, 7(25): 11142–11154
Guo H, Zhang Y, Liang W, et al. An inorganic magnetic fluorescent nanoprobe with favorable biocompatibility for dual-modality bioimaging and drug delivery. Journal of Inorganic Biochemistry, 2019, 192: 72–81
Effenberger F B, Couto R A, Kiyohara P K, et al. Economically attractive route for the preparation of high quality magnetic nanoparticles by the thermal decomposition of iron(III) acetylacetonate. Nanotechnology, 2017, 28(11): 115603
Patsula V, Kosinová L, Lovrić M, et al. Superparamagnetic Fe3O4 nanoparticles: synthesis by thermal decomposition of iron (III) glucuronate and application in magnetic resonance imaging. ACS Applied Materials & Interfaces, 2016, 8(11): 7238–7247
Barbosa I A, de Sousa Filho P C, da Silva D L, et al. Metalloporphyrins immobilized in Fe3O4@SiO2 mesoporous submicrospheres: Reusable biomimetic catalysts for hydrocarbon oxidation. Journal of Colloid and Interface Science, 2016, 469: 296–309
Mumtaz S, Wang S, Hussain S Z, et al. Dopamine coated Fe3O4 nanoparticles as enzyme mimics for the sensitive detection of bacteria. Chemical Communications, 2017, 53(91): 12306–12308
Liu Y, Purich D L, Wu C, et al. Ionic functionalization of hydrophobic colloidal nanoparticles to form ionic nanoparticles with enzyme like properties. Journal of the American Chemical Society, 2015, 137(47): 14952–14958
Wang X, Zhuang J, Peng Q, et al. A general strategy for nanocrystal synthesis. Nature, 2005, 437(7055): 121–124
Cai H, An X, Cui J, et al. Facile hydrothermal synthesis and surface functionalization of polyethyleneimine-coated iron oxide nanoparticles for biomedical applications. ACS Applied Materials & Interfaces, 2013, 5(5): 1722–1731
Bagwe R P, Kanicky J R, Palla B J, et al. Improved drug delivery using microemulsions: rationale, recent progress, and new horizons. Critical Reviews in Therapeutic Drug Carrier Systems, 2001, 18(1): 77–140
Bai F, Wang D, Huo Z, et al. A versatile bottom-up assembly approach to colloidal spheres from nanocrystals. Angewandte Chemie International Edition, 2007, 46(35): 6650–6653
Liu Z L, Wang X, Yao K, et al. Synthesis of magnetite nanoparticles in W/O microemulsion. Journal of Materials Science, 2004, 39(7): 2633–2636
Zhuang L, Zhang W, Zhao Y, et al. Preparation and characterization of Fe3O4 particles with novel nanosheets morphology and magnetochromatic property by a modified solvothermal method. Scientific Reports, 2015, 5(1): 9320
Lastovina T A, Budnyk A P, Kudryavtsev E A, et al. Solvothermal synthesis of Sm3+-doped Fe3O4 nanoparticles. Materials Science and Engineering C, 2017, 80: 110–116
Gao L, Tang Y, Wang C, et al. Highly-efficient amphiphilic magnetic nanocomposites based on a simple sol-gel modification for adsorption of phthalate esters. Journal of Colloid and Interface Science, 2019, 552: 142–152
Zeng Y, Hao R, Xing B, et al. One-pot synthesis of Fe3O4 nanoprisms with controlled electrochemical properties. Chemical Communications, 2010, 46(22): 3920–3922
Carenza E, Barceló V, Morancho A, et al. Rapid synthesis of water-dispersible superparamagnetic iron oxide nanoparticles by a microwave-assisted route for safe labeling of endothelial progenitor cells. Acta Biomaterialia, 2014, 10(8): 3775–3785
Shan D, Deng S, Zhao T, et al. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. Journal of Hazardous Materials, 2016, 305: 156–163
Chang M, Chang Y J, Chao P Y, et al. Exosome purification based on PEG-coated Fe3O4 nanoparticles. PLoS One, 2018, 13 (6): e0199438
Shahabadi N, Falsafi M, Mansouri K. Improving antiproliferative effect of the anticancer drug cytarabine on human promyelocytic leukemia cells by coating on Fe3O4@SiO2 nanoparticles. Colloids and Surfaces B: Biointerfaces, 2016, 141: 213–222
Achilli C, Grandi S, Guidetti G F, et al. Fe3O4@SiO2 core-shell nanoparticles for biomedical purposes: Adverse effects on blood cells. Biomaterials Science, 2016, 4(10): 1417–1421
Wei Y, Yin G, Ma C, et al. Synthesis and cellular compatibility of biomineralized Fe3O4 nanoparticles in tumor cells targeting peptides. Colloids and Surfaces B: Biointerfaces, 2013, 107: 180–188
Wang G, Gao W, Zhang X, et al. Au nanocage functionalized with ultra-small Fe3O4 nanoparticles for targeting T1-T2 dual MRI and CT imaging of tumor. Scientific Reports, 2016, 6: 28258
Felber M, Alberto R. 99mTc radiolabelling of Fe3O4-Au core-shell and Au-Fe3O4 dumbbell-like nanoparticles. Nanoscale, 2015, 7(15): 6653–6660
Gou M, Li S, Zhang L, et al. Facile one-pot synthesis of carbon/ calcium phosphate/Fe3O4 composite nanoparticles for simultaneous imaging and pH/NIR-responsive drug delivery. Chemical Communications, 2016, 52(74): 11068–11071
Shen S, Ding B, Zhang S, et al. Near-infrared light-responsive nanoparticles with thermosensitive yolk-shell structure for multimodal imaging and chemo-photothermal therapy of tumor. Nanomedicine, 2017, 13(5): 1607–1616
Nayak S, Lyon L A. Soft nanotechnology with soft nanoparticles. Angewandte Chemie International Edition, 2005, 44(47): 7686–7708
Tang Z, Zhao X, Zhao T, et al. Magnetic nanoparticles interaction with humic acid: In the presence of surfactants. Environmental Science & Technology, 2016, 50(16): 8640–8648
Dutta B, Shetake N G, Barick B K, et al. pH sensitive surfactant-stabilized Fe3O4 magnetic nanocarriers for dual drug delivery. Colloids and Surfaces B: Biointerfaces, 2018, 162: 163–171
Justin C, Samrot A V, Sruthi D P, et al. Preparation, characterization and utilization of core/shell super paramagnetic iron oxide nanoparticles for curcumin delivery. PLoS One, 2018, 13(7): e0200440
Can H K, Kavlak S, ParviziKhosroshahi S, et al. Preparation, characterization and dynamical mechanical properties of dextrancoated iron oxide nanoparticles (DIONPs). Artificial Cells Nanomedicine and Biotechnology, 2018, 46(2): 421–431
Barrow M, Taylor A, Carrión J C, et al. Co-precipitation of DEAE-dextran coated SPIONs: How synthesis conditions affect particle properties, stem cell labelling and MR contrast. Contrast Media & Molecular Imaging, 2016, 11(5): 362–370
Wu D, Zhu L, Li Y, et al. Superparamagnetic chitosan nanocomplexes for colorectal tumor-targeted delivery of irinotecan. International Journal of Pharmaceutics, 2020, 584: 119394
Soares P I P, Machado D, Laia C, et al. Thermal and magnetic properties of chitosan-iron oxide nanoparticles. Carbohydrate Polymers, 2016, 149: 382–390
Zhang X, Wang Y, Yang S. Simultaneous removal of Co(II) and 1-naphthol by core-shell structured Fe3O4@cyclodextrin magnetic nanoparticles. Carbohydrate Polymers, 2014, 114: 521–529
Xie J, Huang J, Li X, et al. Iron oxide nanoparticle platform for biomedical applications. Current Medicinal Chemistry, 2009, 16 (10): 1278–1294
Wang F, Li X, Li W, et al. Dextran coated Fe3O4 nanoparticles as a near-infrared laser-driven photothermal agent for efficient ablation of cancer cells in vitro and in vivo. Materials Science and Engineering C, 2018, 90: 46–56
Assa F, Jafarizadeh-Malmiri H, Ajamein H, et al. Chitosan magnetic nanoparticles for drug delivery systems. Critical Reviews in Biotechnology, 2017, 37(4): 492–509
Xing H, Zhang S, Bu W, et al. Ultrasmall NaGdF4 nanodots for efficient MR angiography and atherosclerotic plaque imaging. Advanced Materials, 2014, 26(23): 3867–3872
Gupta A K, Wells S. Surface-modified superparamagnetic nanoparticles for drug delivery: Preparation, characterization, and cytotoxicity studies. IEEE Transactions on Nanobioscience, 2004, 3(1): 66–73
Yang G, Ma W, Zhang B, et al. The labeling of stem cells by superparamagnetic iron oxide nanoparticles modified with PEG/ PVP or PEG/PEI. Materials Science and Engineering C, 2016, 62: 384–390
Chen Y, Zhang F, Wang Q, et al. The synthesis of LA-Fe3O4@PDA-PEG-DOX for photothermal therapy-chemotherapy. Dalton Transactions, 2018, 47(7): 2435–2443
Wang L, Wang M, Zhou B, et al. PEGylated reduced-graphene oxide hybridized with Fe3O4 nanoparticles for cancer photothermal-immunotherapy. Journal of Materials Chemistry B: Materials for Biology and Medicine, 2019, 7(46): 7406–7414
Yang C, Guo W, Cui L, et al. pH-responsive magnetic core-shell nanocomposites for drug delivery. Langmuir, 2014, 30(32): 9819–9827
Wei H, Insin N, Lee J, et al. Compact zwitterion-coated iron oxide nanoparticles for biological applications. Nano Letters, 2012, 12(1): 22–25
Liu Y, Chen T, Wu C, et al. Facile surface functionalization of hydrophobic magnetic nanoparticles. Journal of the American Chemical Society, 2014, 136(36): 12552–12555
Wang J J, Lei K F, Han F. Tumor microenvironment: Recent advances in various cancer treatments. European Review for Medical and Pharmacological Sciences, 2018, 22(12): 3855–3864
Zaimy M A, Saffarzadeh N, Mohammadi A, et al. New methods in the diagnosis of cancer and gene therapy of cancer based on nanoparticles. Cancer Gene Therapy, 2017, 24(6): 233–243
Li L, Gao F, Jiang W, et al. Folic acid-conjugated superparamagnetic iron oxide nanoparticles for tumor-targeting MR imaging. Drug Delivery, 2016, 23(5): 1726–1733
Choi H, Choi S R, Zhou R, et al. Iron oxide nanoparticles as magnetic resonance contrast agent for tumor imaging via folate receptor-targeted delivery. Academic Radiology, 2004, 11(9): 996–1004
Yang J, Luo Y, Xu Y, et al. Conjugation of iron oxide nanoparticles with RGD-modified dendrimers for targeted tumor MR imaging. ACS Applied Materials & Interfaces, 2015, 7(9): 5420–5428
Luo Y, Yang J, Yan Y, et al. RGD-functionalized ultrasmall iron oxide nanoparticles for targeted T1-weighted MR imaging of gliomas. Nanoscale, 2015, 7(34): 14538–14546
Zhang H, Li J, Hu Y, et al. Folic acid-targeted iron oxide nanoparticles as contrast agents for magnetic resonance imaging of human ovarian cancer. Journal of Ovarian Research, 2016, 9 (1):19
Cui Y, Zhang C, Luo R, et al. Noninvasive monitoring of early antiangiogenic therapy response in human nasopharyngeal carcinoma xenograft model using MRI with RGD-conjugated ultrasmall superparamagnetic iron oxide nanoparticles. International Journal of Nanomedicine, 2016, 11: 5671–5682
Hirata E, Sahai E. Tumor microenvironment and differential responses to therapy. Cold Spring Harbor Perspectives in Medicine, 2017, 7(7): a026781
Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Letters, 2017, 387: 61–68
Li H, Zhao Y, Jia Y, et al. Covalently assembled dopamine nanoparticle as an intrinsic photosensitizer and pH-responsive nanocarrier for potential application in anticancer therapy. Chemical Communications, 2019, 55(100): 15057–15060
Li E, Yang Y, Hao G, et al. Multifunctional magnetic mesoporous silica nanoagents for in vivo enzyme-responsive drug delivery and MR imaging. Nanotheranostics, 2018, 2(3): 233–242
Yang T, Niu D, Chen J, et al. Biodegradable organosilica magnetic micelles for magnetically targeted MRI and GSH-triggered tumor chemotherapy. Biomaterials Science, 2019, 7(7): 2951–2960
Gao Z, Hou Y, Zeng J, et al. Tumor microenvironment-triggered aggregation of antiphagocytosis 99mTc-labeled Fe3O4 nanoprobes for enhanced tumor imaging in vivo. Advanced Materials, 2017, 29(24): 1701095
Lu J, Sun J, Li F, et al. Highly sensitive diagnosis of small hepatocellular carcinoma using pH-responsive iron oxide nanocluster assemblies. Journal of the American Chemical Society, 2018, 140(32): 10071–10074
Ma T, Hou Y, Zeng J, et al. Dual-ratiometric target-triggered fluorescent probe for simultaneous quantitative visualization of tumor microenvironment protease activity and pH in vivo. Journal of the American Chemical Society, 2018, 140(1): 211–218
Qin M, Peng Y, Xu M, et al. Uniform Fe3O4/Gd2O3-DHCA nanocubes for dual-mode magnetic resonance imaging. Beilstein Journal of Nanotechnology, 2020, 11: 1000–1009
Zhou Z, Huang D, Bao J, et al. A synergistically enhanced T1-T2 dual-modal contrast agent. Advanced Materials, 2012, 24(46): 6223–6228
Lu C, Dong P, Pi L, et al. Hydroxyl-PEG-phosphonic acid-stabilized superparamagnetic manganese oxide-doped iron oxide nanoparticles with synergistic effects for dual-mode MR imaging. Langmuir, 2019, 35(29): 9474–9482
Xu S, Yang F, Zhou X, et al. Uniform PEGylated PLGA microcapsules with embedded Fe3O4 nanoparticles for US/MR dual-modality imaging. ACS Applied Materials & Interfaces, 2015, 7(36): 20460–20468
Cui X, Mathe D, Kovács N, et al. Synthesis, characterization, and application of core-shell Co0.16Fe2.84O4@NaYF4(Yb, Er) and Fe3O4@NaYF4(Yb, Tm) nanoparticle as trimodal (MRI, PET/ SPECT, and optical) imaging agents. Bioconjugate Chemistry, 2016, 27(2): 319–328
Sánchez A, Ovejero Paredes K, Ruiz-Cabello J, et al. Hybrid decorated core@shell Janus nanoparticles as a flexible platform for targeted multimodal molecular bioimaging of cancer. ACS Applied Materials & Interfaces, 2018, 10(37): 31032–31043
Cai W, Guo M, Weng X, et al. Modified green synthesis of Fe3O4@SiO2 nanoparticles for pH responsive drug release. Materials Science and Engineering C, 2020, 112: 110900
Zhang T Y, Li F Y, Xu Q H, et al. Ferrimagnetic nanochains-based mesenchymal stem cell engineering for highly efficient post-stroke recovery. Advanced Functional Materials, 2019, 29 (24): 1900603
Huh Y M, Lee E S, Lee J H, et al. Hybrid nanoparticles for magnetic resonance imaging of target-specificviral gene delivery. Advanced Materials, 2007, 19(20): 3109–3112
Arriortua O K, Insausti M, Lezama L, et al. RGD-functionalized Fe3O4 nanoparticles for magnetic hyperthermia. Colloids and Surfaces B: Biointerfaces, 2018, 165: 315–324
Xu C, Zheng Y, Gao W, et al. Magnetic hyperthermia ablation of tumors using injectable Fe3O4/calcium phosphate cement. ACS Applied Materials & Interfaces, 2015, 7(25): 13866–13875
Li L, Fu S, Chen C, et al. Microenvironment-driven bioelimination of magnetoplasmonic nanoassemblies and their multimodal imaging-guided tumor photothermal therapy. ACS Nano, 2016, 10(7): 7094–7105
Liu Y, Yang Z, Huang X, et al. Glutathione-responsive self-assembled magnetic gold nanowreath for enhanced tumor imaging and imaging-guided photothermal therapy. ACS Nano, 2018, 12(8): 8129–8137
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 11502158, 11632013 and 11802197). The support of the Shanxi Provincial Key Research and Development Project, China (Grant Nos. 201803D421060, 201903D421064 and 201803D421076), the Natural Science Foundation of Shanxi Province, China (201901D111078 and 201901D111077), and the Shanxi Scholarship Council of China (No. HGKY2019037) are also acknowledged with gratitude.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qin, M., Xu, M., Niu, L. et al. Multifunctional modification of Fe3O4 nanoparticles for diagnosis and treatment of diseases: A review. Front. Mater. Sci. 15, 36–53 (2021). https://doi.org/10.1007/s11706-021-0543-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-021-0543-y