Abstract
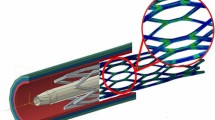
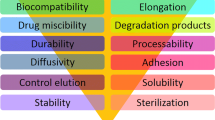
A stent is a medical device designed to serve as a temporary or permanent internal scaffold to maintain or increase the lumen of a body conduit. The researchers and engineers diverted to investigate biodegradable materials due to the limitation of metallic materials in stent application such as stent restenosis which requires prolonged anti platelet therapy, often result in smaller lumen after implantation and obstruct re-stenting treatments. Biomedical implants with temporary function for the vascular intervention are extensively studied in recent years. The rationale for biodegradable stent is to provide the support for the vessel in predicted period of time and then degrading into biocompatible constituent. The degradation of stent makes the re-stenting possible after several months and also ameliorates the vessel wall quality. The present article focuses on the biodegradable materials for the cardiovascular stent. The objective of this review is to describe the possible biodegradable materials for stent and their properties such as design criteria, degradation behavior, drawbacks and advantages with their recent clinical and preclinical trials.
Similar content being viewed by others
References
Arjomand H, Turi Z G, Mc Cormick D, et al. Percutaneous coronary intervention: historical perspectives, current status, and future directions. American Heart Journal, 2003, 146(5): 787–796
Mueller R L, Sanborn T A. The history of interventional cardiology: cardiac catheterization, angioplasty, and related interventions. American Heart Journal, 1995, 129(1): 146–172
Boucher R A, Myler R K, Clark D A, et al. Coronary angiography and angioplasty. Catheterization and Cardiovascular Diagnosis, 1988, 14(4): 269–285
de la Cruz K I, Tsai P I, Cohn W E, et al. Revascularization treatment recommendations based on atherosclerotic disease distribution: coronary artery bypass grafting versus stenting. Current Atherosclerosis Reports, 2008, 10(5): 434–437
Mani G, Feldman M D, Patel D, et al. Coronary stents: a materials perspective. Biomaterials, 2007, 28(9): 1689–1710
Waksman R. Biodegradable stents: they do their job and disappear. The Journal of Invasive Cardiology, 2006, 18(2): 70–74
Bertrand O F, Sipehia R, Mongrain R, et al. Biocompatibility aspects of new stent technology. Journal of the American College of Cardiology, 1998, 32(3): 562–571
Roubin G S, Cannon A D, Agrawal S K, et al. Intracoronary stenting for acute and threatened closure complicating percutaneous transluminal coronary angioplasty. Circulation, 1992, 85 (3): 916–927
Regar E, Sianos G, Serruys P W. Stent development and local drug delivery. British Medical Bulletin, 2001, 59(5): 227–248
Ashby D T, Dangas G, Mehran R, et al. Coronary artery stenting. Catheterization and Cardiovascular Interventions, 2002, 56(1): 83–102
Holzapfel G A, Sommer G, Gasser C T, et al. Determination of layer-specific mechanical properties of human coronary arteries with nonatherosclerotic intimal thickening and related constitutive modeling. American Journal of Physiology- Heart and Circulatory Physiology, 2005, 289(5): H2048–H2058
Frohlich J, Dobiasova M, Lear S, et al. The role of risk factors in the development of atherosclerosis. Critical Reviews in Clinical Laboratory Sciences, 2001, 38(5): 401–440
Robaina S, Jayachandran B, He Y, et al. Platelet adhesion to simulated stented surfaces. Journal of Endovascular Therapy, 2003, 10(5): 978–986
Rogers C, Edelman E R. Endovascular stent design dictates experimental restenosis and thrombosis. Circulation, 1995, 91 (12): 2995–3001
Farb A, Weber D K, Kolodgie F D, et al. Morphological predictors of restenosis after coronary stenting in humans. Circulation, 2002, 105(25): 2974–2980
Wentzel J J, Gijsen F J, Stergiopulos N, et al. Shear stress, vascular remodeling and neointimal formation. Journal of Biomechanics, 2003, 36(5): 681–688
Wentzel J J, Krams R, Schuurbiers J C, et al. Relationship between neointimal thickness and shear stress after Wallstent implantation in human coronary arteries. Circulation, 2001, 103 (13): 1740–1745
Berry J L, Manoach E, Mekkaoui C, et al. Hemodynamics and wall mechanics of a compliance matching stent: in vitro and in vivo analysis. Journal of Vascular & Interventional Radiology, 2002, 13(1): 97–105
Glagov S, Zarins C K, Masawa N, et al. Mechanical functional role of non-atherosclerotic intimal thickening. Frontiers of Medical and Biological Engineering, 1993, 5(1): 37–43
Babapulle M N, Eisenberg M J. Coated stents for the prevention of restenosis: Part II. Circulation, 2002, 106(22): 2859–2866
Rebelo N, Perry M. Finite element analysis for the design of Nitinol medical devices. Minimally Invasive Therapy & Allied Technologies, 2009, 9(2): 75–80
Kastrati A, Dirschinger J, Boekstegers P, et al. Influence of stent design on 1-year outcome after coronary stent placement: a randomized comparison of five stent types in 1,147 unselected patients. Catheterization and Cardiovascular Interventions, 2000, 50(3): 290–297
Griffiths H, Peeters P, Verbist J, et al. Future devices: bioabsorbable stents. The British Journal of Cardiology, 2004, 11: AIC 80–AIC 84
Tominaga R, Kambic H E, Emoto H, et al. Effects of design geometry of intravascular endoprostheses on stenosis rate in normal rabbits. American Heart Journal, 1992, 123(1): 21–28
Gurbel P A, Callahan K P, Malinin A I, et al. Could stent design affect platelet activation? Results of the Platelet Activation in STenting (PAST) study. The Journal of Invasive Cardiology, 2002, 14(10): 584–589
Leimgruber P P, Roubin G S, Anderson H V, et al. Influence of intimal dissection on restenosis after successful coronary angioplasty. Circulation, 1985, 72(3): 530–535
Fischman D L, Leon M B, Baim D S, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. New England Journal of Medicine, 1994, 331(8): 496–501
Nuutinen J P, Clerc C, Reinikainen R, et al. Mechanical properties and in vitro degradation of bioabsorbable selfexpanding braided stents. Journal of Biomaterials Science: Polymer Edition, 2003, 14(3): 255–266
Morton A C, Crossman D, Gunn J. The influence of physical stent parameters upon restenosis. Pathologie Biologie, 2004, 52 (4): 196–205
Lau K W, Johan A, Sigwart U, et al. A stent is not just a stent: Stent construction and design do matter in its clinical performance. Singapore Medical Journal, 2004, 45(7): 305–311
Rogers C D. Optimal stent design for drug delivery. Reviews in Cardiovascular Medicine, 2004, 5(Suppl 2): S9–S15
Bennett M R, O’Sullivan M. Mechanisms of angioplasty and stent restenosis: implications for design of rational therapy. Pharmacology & Therapeutics, 2001, 91(2): 149–166
Tabata Y. Biomaterial technology for tissue engineering applications. Journal of the Royal Society Interface, 2009, 6(Suppl 3): S311–S324
Ramcharitar S, Serruys P W. Fully biodegradable coronary stents: progress to date. American Journal of Cardiovascular Drugs, 2008, 8(5): 305–314
Ormiston J A, Serruys P W, Regar E, et al. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective openlabel trial. Lancet, 2008, 371(9616): 899–907
Seiler H G, Sigel H, Sigel A. Handbook on toxicity of inorganic compounds. Analytica Chimica Acta, 1987, 237: 511
Garg S, Serruys P. Biodegradable stents and non-biodegradable stents. Minerva Cardioangiologica, 2009, 57(5): 537–565
Bourantas C V, Onuma Y, Farooq V, et al. Bioresorbable scaffolds: current knowledge, potentialities and limitations experienced during their first clinical applications. International Journal of Cardiology, 2013, 167(1): 11–21
Heublein B, Rohde R, Kaese V, et al. Biocorrosion of magnesium alloys: a new principle in cardiovascular implant technology? Heart, 2003, 89(6): 651–656
Wiebe J, Nef H M, Hamm C W. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. Journal of the American College of Cardiology, 2014, 64(23): 2541–2551
Iqbal J, Onuma Y, Ormiston J, et al. Bioresorbable scaffolds: rationale, current status, challenges, and future. European Heart Journal, 2014, 35(12): 765–776
Wang Y, Zhang X. Vascular restoration therapy and bioresorbable vascular scaffold. Regenerative Biomaterials, 2014, 1(1): 49–55
Waksman R, Pakala R, Kuchulakanti P K, et al. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheterization and Cardiovascular Interventions, 2006, 68(4): 607–617, discussion 618–619
Ako J, Bonneau H N, Honda Y, et al. Design criteria for the ideal drug-eluting stent. The American Journal of Cardiology, 2007, 100(8B): 3M–9M
Kitabata H, Waksman R, Warnack B. Bioresorbable metal scaffold for cardiovascular application: current knowledge and future perspectives. Cardiovascular Revascularization Medicine, 2014, 15(2): 109–116
Di Mario C, Griffiths H, Goktekin O, et al. Drug-eluting bioabsorbable magnesium stent. Journal of Interventional Cardiology, 2004, 17(6): 391–395
Ruiz-García J, Refoyo E, Cuesta-López E, et al. Comparative results between metal stent and bioresorbable scaffold at two years postimplantation. Revista Espanola de Cardiologia (English Edition), 2014, 67(1): 66–68
Echeverri D, Cabrales J R. Terapia de restauración vascular con plataformas biorreabsorbibles. La cuarta revolución. Revista Colombiana de Cardiología, 2014, 21(4): 231–240
Puppi D, Chiellini F, Piras A M, et al. Polymeric materials for bone and cartilage repair. Progress in Polymer Science, 2010, 35 (4): 403–440
Williams D F. Biodegradation of surgical polymers. Journal of Materials Science, 1982, 17(5): 1233–1246
Helmus M N, Gibbons D F, Cebon D. Biocompatibility: meeting a key functional requirement of next-generation medical devices. Toxicologic Pathology, 2008, 36(1): 70–80
Chen G, Ushida T, Tateishi T. Scaffold design for tissue engineering. Macromolecular Bioscience, 2002, 2(2): 67–77
Freier T. Biopolyesters in tissue engineering applications. Advances in Polymer Science, 2006, 203(1): 1–61
Sokolsky-Papkov M, Langer R, Domb A J. Synthesis of aliphatic polyesters by polycondensation using inorganic acid as catalyst. Polymers for Advanced Technologies, 2011, 22(5): 502–511
Tamai H, Igaki K, Kyo E, et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation, 2000, 102(4): 399–404
Ceonzo K, Gaynor A, Shaffer L, et al. Polyglycolic acid-induced inflammation: role of hydrolysis and resulting complement activation. Tissue Engineering, 2006, 12(2): 301–308
Brown D A, Lee EW, Loh C T, et al. A new wave in treatment of vascular occlusive disease: biodegradable stents–clinical experience and scientific principles. Journal of Vascular and Interventional Radiology, 2009, 20(3): 315–324
Martin O, Averous L. Poly (lactic acid): plasticization and properties of biodegradable multiphase systems. Polymer, 2001, 42(14): 6209–6219
Sabir M I, Xu X, Li L. A review on biodegradable polymeric materials for bone tissue engineering applications. Journal of Materials Science, 2009, 44(21): 5713–5724
Pamula E, Menaszek E. In vitro and in vivo degradation of poly (L-lactide-co-glycolide) films and scaffolds. Journal of Materials Science: Materials in Medicine, 2008, 19(5): 2063–2070
Leenslag JW, Pennings A J, Bos R R, et al. Resorbable materials of poly(L-lactide): VII. In vivo and in vitro degradation. Biomaterials, 1987, 8(4): 311–314
Grabow N, Schlun M, Sternberg K, et al. Mechanical properties of laser cut poly(L-lactide) micro-specimens: implications for stent design, manufacture, and sterilization. Journal of Biomechanical Engineering, 2005, 127(1): 25–31
Stack R S, Califf R M, Phillips H R, et al. Interventional cardiac catheterization at Duke Medical Center. American Journal of Cardiology, 1988, 62(10 Pt 2): 3F–24F
Venkatraman S, Boey F, Lao L L. Implanted cardiovascular polymers: Natural, synthetic and bio-inspired. Progress in Polymer Science, 2008, 33(9): 853–874
Piao L, Deng M, Chen X, et al. Ring-opening polymerization of e-caprolactone and L-lactide using organic amino calcium catalyst. Polymer, 2003, 44(8): 2331–2336
Bourantas C V, Zhang Y, Farooq V, et al. Bioresorbable scaffolds: current evidence and ongoing clinical trials. Current Cardiology Reports, 2012, 14(5): 626–634
Lepu Medical. NeoVas biodegradable scaffold ongoing clinical trials overview.[EB/OL], 2015, http://finance.qq.com/a/ 20151127/030523.htm
MicroPort®. Firesorb bioresorbable rapamycin target eluting coronary scaffold system completes first successful implantation in the first FIM clinical trial.[EB/OL], 2015, http://www. microportmedical.com/en/media.php?curr_page = news_details& id = 339
Engelberg I, Kohn J. Physico-mechanical properties of degradable polymers used in medical applications: a comparative study. Biomaterials, 1991, 12(3): 292–304
Pitt C G, Gu Z W. Modification of the rates of chain cleavage of poly(e-caprolactone) and related polyesters in the solid state. Journal of Controlled Release, 1987, 4(4): 283–292
Heller J. Development of poly(ortho esters): a historical overview. Biomaterials, 1990, 11(9): 659–665
van der Giessen W J, Lincoff A M, Schwartz R S, et al. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation, 1996, 94(7): 1690–1697
Gao R, Shi R, Qiao S, et al. A novel polymeric local heparin delivery stent: Initial experimental study. Journal of the American College of Cardiology, 1996, 27(2): 85–86
Susawa T, Shiraki K, Shimizu Y. Biodegradable intracoronary stents in adult dogs. Journal of the American College of Cardiology, 1993, 21: 483A
Tsuji T, Tamai H, Igaki K, et al. Biodegradable stents as a platform to drug loading. International Journal of Cardiovascular Interventions, 2003, 5(1): 13–16
Zidar J, Lincoff A, Stack R. Biodegradable stents. Textbook of Interventional Cardiology, 1994, 2: 787–802
Ye Y W, Landau C, Meidell R S, et al. Improved bioresorbable microporous intravascular stents for gene therapy. ASAIO Journal, 1996, 42(5): M823–M827
Heller J, Barr J, Ng S Y, et al. Poly(ortho esters)–their development and some recent applications. European Journal of Pharmaceutics and Biopharmaceutics, 2000, 50(1): 121–128
Capancioni S, Schwach-Abdellaoui K, Kloeti W, et al. In vitro monitoring of poly (ortho ester) degradation by electron paramagnetic resonance imaging. Macromolecules, 2003, 36 (16): 6135–6141
Göpferich A. Mechanisms of polymer degradation and erosion. Biomaterials, 1996, 17(2): 103–114
Hofmann D, Entrialgo-Castaño M, Kratz K, et al. Knowledgebased approach towards hydrolytic degradation of polymerbased biomaterials. Advanced Materials, 2009, 21(32–33): 3237–3245
Heller J. Poly (ortho esters). Berlin Heidelberg: Springer, 1993, 41–92
Heller J, Penhale DW, Fritzinger B K, et al. Controlled release of contraceptive steroids from biodegradable poly (ortho esters). Contraceptive Delivery Systems, 1983, 4(1): 43–53
Shih C, Higuchi T, Himmelstein K J. Drug delivery from catalysed erodible polymeric matrices of poly(ortho ester)s. Biomaterials, 1984, 5(4): 237–240
Baei M S, Najafpour G D, Younesi H, et al. Poly(3- hydroxybutyrate) synthesis by cupriavidus necator DSMZ 545 utilizing various carbon sources. World Applied Sciences Journal, 2009, (2): 157–161
Holland S J, Jolly A M, Yasin M, et al. Polymers for biodegradable medical devices: II. Hydroxybutyrate–hydroxyvalerate copolymers: hydrolytic degradation studies. Biomaterials, 1987, 8(4): 289–295
Wang H T, Palmer H, Linhardt R J, et al. Degradation of poly (ester) microspheres. Biomaterials, 1990, 11(9): 679–685
Zhao K, Deng Y, Chen G Q. Effects of surface morphology on the biocompatibility of polyhydroxyalkanoates. Biochemical Engineering Journal, 2003, 16(2): 115–123
Gogolewski S, Jovanovic M, Perren S M, et al. Tissue response and in vivo degradation of selected polyhydroxyacids: polylactides (PLA), poly(3-hydroxybutyrate) (PHB), and poly(3-hydroxybutyrate- co-3-hydroxyvalerate) (PHB/VA). Journal of Biomedical Materials Research, 1993, 27(9): 1135–1148
Unverdorben M, Spielberger A, Schywalsky M, et al. A polyhydroxybutyrate biodegradable stent: preliminary experience in the rabbit. Cardiovascular and Interventional Radiology, 2002, 25(2): 127–132
Domb A J, Amselem S, Shah J, et al. Polyanhydrides: Synthesis and characterization. Berlin Heidelberg: Springer, 1993, 93–141
Lucas N, Bienaime C, Belloy C, et al. Polymer biodegradation: mechanisms and estimation techniques. Chemosphere, 2008, 73 (4): 429–442
Davies M C, Shakesheff K M, Shard A G, et al. Surface analysis of biodegradable polymer blends of poly(sebacic anhydride) and poly(DL-lactic acid). Macromolecules, 1996, 29(6): 2205–2212
Uhrich K E, Gupta A, Thomas T T, et al. Synthesis and characterization of degradable poly(anhydride-co-imides). Macromolecules, 1995, 28(7): 2184–2193
Chasin M, Domb A, Ron E, et al. Polyanhydrides as drug delivery systems. In: Chasin M, Langer R, eds. Biodegradable Polymers as Drug Delivery Systems. New York: Marcel Dekker, 1990, 45: 43–70
Laurencin C, Domb A, Morris C, et al. Poly(anhydride) administration in high doses in vivo: studies of biocompatibility and toxicology. Journal of Biomedical Materials Research, 1990, 24(11): 1463–1481
Jabara R. Poly-anhydride based on salicylic acid and adipic acid anhydride. Barcelona, Spain: EuroPCR, 2009
Wang S, Lu L, Yaszemski M J. Bone-tissue-engineering material poly(propylene fumarate): correlation between molecular weight, chain dimensions, and physical properties. Biomacromolecules, 2006, 7(6): 1976–1982
Shung A K, Timmer M D, Jo S, et al. Kinetics of poly(propylene fumarate) synthesis by step polymerization of diethyl fumarate and propylene glycol using zinc chloride as a catalyst. Journal of Biomaterials Science: Polymer Edition, 2002, 13(1): 95–108
Fisher J P, Dean D, Mikos A G. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly(propylene fumarate) biomaterials. Biomaterials, 2002, 23(22): 4333–4343
Suggs L J, Krishnan R S, Garcia C A, et al. In vitro and in vivo degradation of poly(propylene fumarate-co-ethylene glycol) hydrogels. Journal of Biomedical Materials Research, 1998, 42 (2): 312–320
Herold D A, Keil K, Bruns D E. Oxidation of polyethylene glycols by alcohol dehydrogenase. Biochemical Pharmacology, 1989, 38(1): 73–76
Gilding D K, Reed A M. Biodegradable polymers for use in surgery–poly(ethylene oxide) poly(ethylene terephthalate) (PEO/PET) copolymers: 1. Polymer, 1979, 20(12): 1454–1458
Mody P C, Wilkes G L, Wagener K B, et al. Structure–property relationships of a new series of segmented polyether–polyester copolymers. Journal of Applied Polymer Science, 1981, 26(9): 2853–2878
Pathak C P, Sawhney A S, Quinn C P, et al. Polyimidepolyethylene glycol block copolymers: synthesis, characterization, and initial evaluation as a biomaterial. Journal of Biomaterials Science: Polymer Edition, 1994, 6(4): 313–323
Zhu K J, Lin X, Yang S. Preparation and properties of D, Llactide and ethylene oxide copolymer: A modifying biodegradable polymeric material. Journal of Polymer Science Part C: Polymer Letters, 1986, 24(7): 331–337
Sawhney A S, Pathak C P, Hubbell J A. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(a- hydroxy acid) diacrylate macromers. Macromolecules, 1993, 26 (4): 581–587
Hill-West J L, Chowdhury S M, Slepian M J, et al. Inhibition of thrombosis and intimal thickening by in situ photopolymerization of thin hydrogel barriers. Proceedings of the National Academy of Sciences of the United States of America, 1994, 91 (13): 5967–5971
Suggs L J, Shive M S, Garcia C A, et al. In vitro cytotoxicity and in vivo biocompatibility of poly(propylene fumarate-co-ethylene glycol) hydrogels. Journal of Biomedical Materials Research, 1999, 46(1): 22–32
Kohn J, Langer R. Polymerization reactions involving the side chains of a-L-amino acids. Journal of the American Chemical Society, 1987, 109(3): 817–820
Ertel S I, Kohn J. Evaluation of a series of tyrosine-derived polycarbonates as degradable biomaterials. Journal of Biomedical Materials Research, 1994, 28(8): 919–930
Tangpasuthadol V, Pendharkar S M, Kohn J. Hydrolytic degradation of tyrosine-derived polycarbonates, a class of new biomaterials. Part I: study of model compounds. Biomaterials, 2000, 21(23): 2371–2378
Tangpasuthadol V, Pendharkar S M, Peterson R C, et al. Hydrolytic degradation of tyrosine-derived polycarbonates, a class of new biomaterials. Part II: 3-yr study of polymeric devices. Biomaterials, 2000, 21(23): 2379–2387
Pulapura S, Kohn J. Tyrosine-derived polycarbonates: backbonemodified “pseudo”-poly (amino acids) designed for biomedical applications. Biopolymers, 1992, 32(4): 411–417
Bourke S L, Kohn J, Dunn M G. Preliminary development of a novel resorbable synthetic polymer fiber scaffold for anterior cruciate ligament reconstruction. Tissue Engineering, 2004, 10 (1–2): 43–52
Bailey L O, Becker M L, Stephens J S, et al. Cellular response to phase-separated blends of tyrosine-derived polycarbonates. Journal of Biomedical Materials Research Part A, 2006, 76(3): 491–502
Strandberg E, Zeltinger J, Schulz D G, et al. Late positive remodeling and late lumen gain contribute to vascular restoration by a non-drug eluting bioresorbable scaffold: a four-year intravascular ultrasound study in normal porcine coronary arteries. Circulation: Cardiovascular Interventions, 2012, 5(1): 39–46
Grube E. The REVA Tyrosine-derived polycarbonate bioabsorbable stent: final results from the RESORB First-in-man clinical trial and next generation designs. Transcatheter Cardiovascular Therapeutics, 2008
Witte F. Reprint of: The history of biodegradable magnesium implants: A review. Acta Biomaterialia, 2015, 23(Supp l): S28–S40
Song G, Song S Z. A possible biodegradable magnesium implant material. Advanced Engineering Materials, 2007, 9(4): 298–302
Vormann J. Magnesium: nutrition and metabolism. Molecular Aspects of Medicine, 2003, 24(1–3): 27–37
Mult E, Haferkamp H, Niemeyer M, et al. Laser and electron beam welding of magnesium materials. Welding and Cutting, 2000, 52(8): 178–180
Marya M, Edwards G. The laser welding of magnesium alloy AZ91. Welding in the World, 2000, 44(2): 31–37
Mordike B, Ebert T. Magnesium: properties–applications–potential. Materials Science and Engineering A, 2001, 302(1): 37–45
Aghion E, Bronfin B. Magnesium alloys development towards the 21st century. Materials Science Forum, 2000, 350–351: 19–30
Pastor M, Zhao H, Debroy T. Continuous wave-Nd: yttrium–aluminum–garnet laser welding of AM60B magnesium alloy. Journal of Laser Applications, 2000, 12(3): 91–100
Marya M, Edwards G, Marya S, et al. Fundamentals in the fusion welding of magnesium and its alloys. Proceedings of the Seventh JWS International Symposium, Kobe, 2001, 597–602
Waksman R, Pakala R, Kuchulakanti P K, et al. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheterization and Cardiovascular Interventions, 2006, 68(4): 607–617, discussion 618–619
Waksman R, Pakala R, Hellinga D, et al. Effect of bioabsorbable magnesium alloy stent on neointimal formation in a porcine coronary model. European Heart Journal, 2005, 26: 417
Waksman R, Pakala R, Okabe T, et al. Efficacy and safety of absorbable metallic stents with adjunct intracoronary beta radiation in porcine coronary arteries. Journal of Interventional Cardiology, 2007, 20(5): 367–372
Friedrich H E, Mordike B L. Magnesium Technology. Berlin: Springer, 2006, 788
Erbel R, Di Mario C, Bartunek J, et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet, 2007, 369(9576): 1869–1875
Peeters P, Bosiers M, Verbist J, et al. Preliminary results after application of absorbable metal stents in patients with critical limb ischemia. Journal of Endovascular Therapy, 2005, 12(1): 1–5
Schranz D, Zartner P, Michel-Behnke I, et al. Bioabsorbable metal stents for percutaneous treatment of critical recoarctation of the aorta in a newborn. Catheterization and Cardiovascular Interventions, 2006, 67(5): 671–673
Bach F W, Schaper M, Jaschik C. Influence of lithium on hcp magnesium alloys. Materials Science Forum, 2003, 419–422: 1037–1042
Kaese V, Niemeyer M, Tai P T, et al. Korrosionsschützendes Legieren von Magnesiumbasiswerkstoffen. Teil 1: Dynamische Alkalisierung der Grenzschicht-Tertiäre Legierungssysteme. Materials & Corrosion, 1999, 50(4): 191–198
Magnesium Elektron Datasheet. WE43. Magnesium Elektron, 2005
Günter N, Kohei K, Kenji H, et al. Magnesium-Based Alloys. Wiley-VCH Verlag GmbH & Co. KGaA, 2006
Nagels J, Stokdijk M, Rozing P M. Stress shielding and bone resorption in shoulder arthroplasty. Journal of Shoulder and Elbow Surgery, 2003, 12(1): 35–39
Park J B, Bronzino J D. Biomaterials: Principles and Applications. CRC Press, 2003
Clark G C, Williams D F. The effects of proteins on metallic corrosion. Journal of Biomedical Materials Research, 1982, 16 (2): 125–134
Waksman R, Pakala R, Kuchulakanti P K, et al. Safety and efficacy of bioabsorbable magnesium alloy stents in porcine coronary arteries. Catheterization and Cardiovascular Interventions, 2006, 68(4): 607–617, discussion 618–619
Waksman R, Pakala R, Okabe T, et al. Efficacy and safety of absorbable metallic stents with adjunct intracoronary beta radiation in porcine coronary arteries. Journal of Interventional Cardiology, 2007, 20(5): 367–372
Kitabata H, Waksman R, Warnack B. Bioresorbable metal scaffold for cardiovascular application: current knowledge and future perspectives. Cardiovascular Revascularization Medicine, 2014, 15(2): 109–116
Zartner P, Cesnjevar R, Singer H, et al. First successful implantation of a biodegradable metal stent into the left pulmonary artery of a preterm baby. Catheterization and Cardiovascular Interventions, 2005, 66(4): 590–594
Schranz D, Zartner P, Michel-Behnke I, et al. Bioabsorbable metal stents for percutaneous treatment of critical recoarctation of the aorta in a newborn. Catheterization and Cardiovascular Interventions, 2006, 67(5): 671–673
Zartner P, Buettner M, Singer H, et al. First biodegradable metal stent in a child with congenital heart disease: evaluation of macro and histopathology. Catheterization and Cardiovascular Interventions, 2007, 69(3): 443–446
McMahon C J, Oslizlok P, Walsh K P. Early restenosis following biodegradable stent implantation in an aortopulmonary collateral of a patient with pulmonary atresia and hypoplastic pulmonary arteries. Catheterization and Cardiovascular Interventions, 2007, 69(5): 735–738
Morice M C, Serruys P W, Sousa E J. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. The New England Journal of Medicine, 2002, 346(23): 1773–1780
Se&Co B. CORRECTING & REPLACING BIOTRONIK announces positive 6-month results for dreams, the pioneering drug-eluting absorbable metal scaffold. Biomedical Market Newsletter, 5/17/2011, 257
Hentze M W, Muckenthaler M U, Andrews N C. Balancing acts: molecular control of mammalian iron metabolism. Cell, 2004, 117(3): 285–297
May T, Mueller P P, Weich H, et al. Establishment of murine cell lines by constitutive and conditional immortalization. Journal of Biotechnology, 2005, 120(1): 99–110
Hermawan H, Alamdari H, Mantovani D, et al. Iron–manganese: new class of metallic degradable biomaterials prepared by powder metallurgy. Powder Metallurgy, 2008, 51(1): 38–45
Peuster M, Hesse C, Schloo T, et al. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials, 2006, 27(28): 4955–4962
Moravej M, Prima F, Fiset M, et al. Electroformed iron as new biomaterial for degradable stents: development process and structure–properties relationship. Acta Biomaterialia, 2010, 6(5): 1726–1735
Zhu S, Huang N, Xu L, et al. Biocompatibility of Fe–O films synthesized by plasma immersion ion implantation and deposition. Surface and Coatings Technology, 2009, 203(10–11): 1523–1529
Liu B, Zheng Y F, Ruan L. In vitro investigation of Fe30Mn6Si shape memory alloy as potential biodegradable metallic material. Materials Letters, 2011, 65(3): 540–543
Nie F L, Zheng Y F, Wei S C, et al. In vitro corrosion, cytotoxicity and hemocompatibility of bulk nanocrystalline pure iron. Biomedical Materials, 2010, 5(6): 065015
Mueller P P, May T, Perz A, et al. Control of smooth muscle cell proliferation by ferrous iron. Biomaterials, 2006, 27(10): 2193–2200
Francis A, Yang Y, Virtanen S, et al. Iron and iron-based alloys for temporary cardiovascular applications. Journal of Materials Science: Materials in Medicine, 2015, 26(3): 138
Peuster M, Wohlsein P, Brügmann M, et al. A novel approach to temporary stenting: degradable cardiovascular stents produced from corrodible metal-results 6–18 months after implantation into New Zealand white rabbits. Heart, 2001, 86(5): 563–569
Schinhammer M, Hänzi A C, Löffler J F, et al. Design strategy for biodegradable Fe-based alloys for medical applications. Acta Biomaterialia, 2010, 6(5): 1705–1713
Hermawan H, Purnama A, Dube D, et al. Fe–Mn alloys for metallic biodegradable stents: degradation and cell viability studies. Acta Biomaterialia, 2010, 6(5): 1852–1860
Moravej M, Purnama A, Fiset M, et al. Electroformed pure iron as a new biomaterial for degradable stents: in vitro degradation and preliminary cell viability studies. Acta Biomaterialia, 2010, 6 (5): 1843–1851
Liu B, Zheng Y F. Effects of alloying elements (Mn, Co, Al, W, Sn, B, C and S) on biodegradability and in vitro biocompatibility of pure iron. Acta Biomaterialia, 2011, 7(3): 1407–1420
Lin WJ, Zhang D Y, Zhang G, et al. Design and characterization of a novel biocorrodible iron-based drug-eluting coronary scaffold. Materials & Design, 2015, 91: 72–79
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, LD., Li, Z., Pan, Y. et al. A review on biodegradable materials for cardiovascular stent application. Front. Mater. Sci. 10, 238–259 (2016). https://doi.org/10.1007/s11706-016-0344-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-016-0344-x