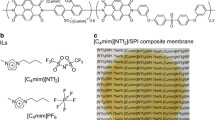
Abstract
Ionic liquid (IL)/polyimide (PI) composite membranes demonstrate promise for use in CO2 separation applications. However, few studies have focused on the microscopic mechanism of CO2 in these composite systems, which is important information for designing new membranes. In this work, a series of systems of CO2 in 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide composited with 4,4-(hexafluoroisopropylidene) diphthalic anhydride (6FDA)-based PI, 6FDA-2,3,5,6-tetramethyl-1,4-phenylene-diamine, at different IL concentrations were investigated by all-atom molecular dynamics simulation. The formation of IL regions in PI was found, and the IL regions gradually became continuous channels with increasing IL concentrations. The analysis of the radial distribution functions and hydrogen bond numbers demonstrated that PI had a stronger interaction with cations than anions. However, the hydrogen bonds among PI chains were destroyed by the addition of IL, which was favorable for transporting CO2. Furthermore, the self-diffusion coefficient and free energy barrier suggested that the diffusion coefficient of CO2 decreased with increasing IL concentrations up to 35 wt-% due to the decrease of the fractional free volume of the composite membrane. However, the CO2 self-diffusion coefficients increased when the IL contents were higher than 35 wt-%, which was attributed to the formation of continuous IL domain that benefitted the transportation of CO2.

Similar content being viewed by others
References
Deng L Y, Kvamsdal H. CO2 capture: challenges and opportunities. Green Energy & Environment, 2016, 1(3): 179–179
Quale S, Rohling V. The European carbon dioxide capture and storage laboratory infrastructure (ECCSEL). Green Energy & Environment, 2016, 1(3): 180–194
Boothandford M E, Abanades J C, Anthony E J, Blunt M J, Brandani S, Dowell N M, Fernandez J R, Ferrari M, Gross R, Hallett J P. Carbon capture and storage update. Energy & Environmental Science, 2014, 7(1): 130–189
Vitillo J G, Smit B, Gagliardi L. Introduction: carbon capture and separation. Chemical Reviews, 2017, 117(14): 9521–9523
Li M, Jiang X, He G. Application of membrane separation technology in postcombustion carbon dioxide capture process. Frontiers of Chemical Science and Engineering, 2014, 8(2): 233–239
Kanehashi S, Scholes C A. Perspective of mixed matrix membranes for carbon capture. Frontiers of Chemical Science and Engineering, 2020, 14(3): 1–10
Pandiyan S, Brown D, Neyertz S, van der Vegt N F A. Carbon dioxide solubility in three fluorinated polyimides studied by molecular dynamics simulations. Macromolecules, 2010, 43(5): 2605–2621
Tong H, Hu C, Yang S, Ma Y, Guo H, Fan L. Preparation of fluorinated polyimides with bulky structure and their gas separation performance correlated with microstructure. Polymer, 2015, 69: 138–147
O’Harra K E, Kammakakam I, Devriese E M, Noll D M, Bara J E, Jackson E M. Synthesis and performance of 6FDA-based polyimide-ionenes and composites with ionic liquids as gas separation membranes. Membranes, 2019, 9(7): 79–96
Kanehashi S, Kishida M, Kidesaki T, Shindo R, Sato S, Miyakoshi T, Nagai K. CO2 separation properties of a glassy aromatic polyimide composite membranes containing high-content 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ionic liquid. Journal of Membrane Science, 2013, 430: 211–222
Weigelt F, Escorihuela S, Descalzo A, Tena A, Escolastico S, Shishatskiy S, Serra J M, Brinkmann T. Novel polymeric thin-film composite membranes for high-temperature gas separations. Membranes, 2019, 9(4): 51–64
Zhang X, Feng H, Liu Z, Wang W, Maginn E J. Absorption of CO2 in the ionic liquid 1-n-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate ([Hmim][FEP]): a molecular view by computer simulations. Journal of Physical Chemistry B, 2009, 113 (21): 7591–7598
Zhang X, Liu Z, Wang W. Screening of ionic liquids to capture CO2 by COSMO-RS and experiments. AIChE Journal. American Institute of Chemical Engineers, 2008, 54(10): 2717–2728
Haghtalab A, Shojaeian A. High pressure measurement and thermodynamic modelling of the solubility of carbon dioxide in N-methyldiethanolamine and 1-butyl-3-methylimidazolium acetate mixture. Journal of Chemical Thermodynamics, 2015, 81: 237–244
Jalili A H, Mehdizadeh A, Shokouhi M, Sakhaeinia H, Taghikhani V. Solubility of CO2 in 1-(2-hydroxyethyl)-3-methylimidazolium ionic liquids with different anions. Journal of Chemical Thermodynamics, 2010, 42(6): 787–791
Lei X, Xu Y, Zhu L, Wang X. Highly efficient and reversible CO2 capture through 1,1,3,3-tetramethylguanidinium imidazole ionic liquid. RSC Advances, 2014, 4(14): 7052–7054
Wang J, Zeng S, Huo F, Shang D, He H, Bai L, Zhang X, Li J. Metal chloride anion-based ionic liquids for efficient separation of NH3. Journal of Cleaner Production, 2019, 206: 661–669
Li P, Coleman M R. Synthesis of room temperature ionic liquids based random copolyimides for gas separation applications. European Polymer Journal, 2013, 49(2): 482–491
Mittenthal M S, Flowers B S, Bara J E, Whitley J W, Spear S K, Roveda J D, Wallace D A, Shannon M S, Holler R, Martens R, Daly D T. Ionic polyimides: hybrid polymer architectures and composites with ionic liquids for advanced gas separation membranes. Industrial & Engineering Chemistry Research, 2017, 56(17): 5055–5069
Ito A, Yasuda T, Ma X, Watanabe M. Sulfonated polyimide/ionic liquid composite membranes for carbon dioxide separation. Polymer Journal, 2017, 49(9): 671–676
Monteiro B, Nabais A R, Almeida Paz F A, Cabrita L, Branco L C, Marrucho I M, Neves L A, Pereira C C L. Membranes with a low loading of metal-organic framework-supported ionic liquids for CO2/N2 separation in CO2 capture. Energy Technology (Weinheim), 2017, 5(12): 2158–2162
Balçık M, Ahunbay M G. Prediction of CO2-induced plasticization pressure in polyimides via atomistic simulations. Journal of Membrane Science, 2018, 547: 146–155
Zhang X, Liu Z, Liu X. Understanding the interactions between tris (pentafluoroethyl)-trifluorophosphate-based ionic liquid and small molecules from molecular dynamics simulation. Science China. Chemistry, 2012, 55(8): 1557–1565
Lourenco T C, Coelho M F, Ramalho T C, Van Der Spoel D, Costa L T. Insights on the solubility of CO2 in 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide from the microscopic point of view. Environmental Science & Technology, 2013, 47(13): 7421–7429
Abedini A, Crabtree E, Bara J E, Turner C H. Molecular simulation of ionic polyimides and composites with ionic liquids as gasseparation membranes. Langmuir, 2017, 33(42): 11377–11389
Spoel D V D, Lindahl E, Hess B, Groenhof G, Berendsen H J C. GROMACS: fast, flexible, and free. Journal of Computational Chemistry, 2005, 26(16): 1701–1718
Hess B, Kutzner C, Van Der Spoel D, Lindahl E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. Journal of Chemical Theory and Computation, 2008, 4(3): 435–447
Liu Z P, Huang S P, Wang W C. A refined force field for molecular simulation of imidazolium-based ionic liquids. Journal of Physical Chemistry B, 2004, 108(34): 12978–12989
Lopes J N C, Pádua A A H. Molecular force field for ionic liquids composed of triflate or bistriflylimide anions. Journal of Physical Chemistry B, 2004, 108(43): 16893–16898
Tanis I, Brown D, Neyertz S J, Heck R, Mercier R. A comparison of homopolymer and block copolymer structure in 6FDA-based polyimides. Physical Chemistry Chemical Physics, 2014, 16(42): 23044–23055
Wang J, Wolf R M, Caldwell J W, Kollman P A, Case D A. Development and testing of a general amber force field. Journal of Computational Chemistry, 2004, 25(9): 1157–1174
Martínez L, Andrade R, Birgin E G, Martínez J M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. Journal of Computational Chemistry, 2009, 30(13): 2157–2164
Essmann U, Perera L, Berkowitz M L, Darden T, Lee H, Pedersen L G. A smooth particle mesh Ewald method. Journal of Chemical Physics, 1995, 103(19): 8577–8593
Hess B, Bekker H, Berendsen H J C, Fraaije J G E M. LINCS: a linear constraint solver for molecular simulations. Journal of Chemical Theory and Computation, 1997, 18(12): 1463–1472
Parrinello M, Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method. Journal of Applied Physics, 1981, 52(12): 7182–7190
Braga C, Travis K P. A configurational temperature Nosé-Hoover thermostat. Journal of Computational Physics, 2005, 123(13): 134101–134116
Shi W, Maginn E J. Atomistic simulation of the absorption of carbon dioxide and water in the ionic liquid 1-n-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [hmim] [Tf2N]. Journal of Physical Chemistry B, 2008, 112(7): 2045–2055
Torrie G M, Valleau J P. Nonphysical sampling distributions in Monte Carlo free-energy estimation: umbrella sampling. Journal of Computational Physics, 1977, 23(2): 187–199
Kumar S, Rosenberg J M, Bouzida D, Swendsen R H, Kollman P A. The weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. Journal of Computational Chemistry, 1992, 13(8): 1011–1021
Wang Y, Wang C, Zhang Y, Huo F, He H, Zhang S. Molecular insights into the regulatable interfacial property and flow behavior of confined ionic liquids in graphene nanochannels. Small, 2019, 15 (29): 1804508–1804518
Sun D L, Zhou J. Ionic liquid confined in nafion: toward molecular-level understanding. AIChE Journal. American Institute of Chemical Engineers, 2013, 59(7): 2630–2639
Song T, Zhang X, Li Y, Jiang K, Zhang S, Cui X, Bai L. Separation efficiency of CO2 in ionic liquids/poly(vinylidene fluoride) composite membrane: a molecular dynamics study. Industrial & Engineering Chemistry Research, 2019, 58(16): 6887–6898
Zhang X, Huo F, Liu X, Dong K, He H, Yao X, Zhang S. Influence of microstructure and interaction on viscosity of ionic liquids. Industrial & Engineering Chemistry Research, 2015, 54(13): 3505–3514
Dong K, Liu X, Dong H, Zhang X, Zhang S. Multiscale studies on ionic liquids. Chemical Reviews, 2017, 117(10): 6636–6695
Bondi A. Van der Waals volumes and radii. Journal of Physical Chemistry, 1964, 68(3): 441–451
Lee W M. Selection of barrier materials from molecular-structure. Polymer Engineering and Science, 1980, 20(1): 65–69
Tanaka K, Kita H, Okano M, Okamoto K. Permeability and permselectivity of gases in fluorinated and non-fluorinated polyimides. Polymer, 1992, 33(3): 585–592
Cheng H, Zhang J, Qi Z. Effects of interaction with sulphur compounds and free volume in imidazolium-based ionic liquid on desulphurisation: a molecular dynamics study. Molecular Simulation, 2018, 44(1): 55–62
Cantley L, Swett J L, Lloyd D, Cullen D A, Zhou K, Bedworth P V, Heise S, Rondinone A J, Xu Z P, Sinton S, Bunch J S. Voltage gated inter-cation selective ion channels from graphene nanopores. Nanoscale, 2019, 11(20): 9856–9861
Zhou K, Xu Z P. Ion permeability and selectivity in composite nanochannels: engineering through the end effects. Journal of Physical Chemistry C, 2020, 124(8): 4890–4898
Acknowledgements
This work was supported by the National Key R&D Program of China (Grant No. 2018YFB0605802), the National Natural Science Foundation of China (Grant Nos. 51674234, 21978293, U1704251), “Transformational Technologies for Clean Energy and Demonstration”, Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA 21030500), NSFC-NRCT joint project (Grant No. 51661145012), and CAS Pioneer Hundred Talents Program.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
You, L., Guo, Y., He, Y. et al. Molecular level understanding of CO2 capture in ionic liquid/polyimide composite membrane. Front. Chem. Sci. Eng. 16, 141–151 (2022). https://doi.org/10.1007/s11705-020-2009-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-020-2009-7