Abstract
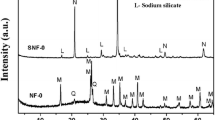
The coal fly ash produced by gasification is estimated to be over 80 million ton per year in China by 2021. It has mainly been disposed as solid waste by landfill. There is lack of study focused on its utilization. In this paper, the coal fly ash produced by gasification was at first analyzed and then applied to synthesize zeolite as an adsorbent. The effects of synthesis conditions on the cation exchange capacity (CEC) of zeolite were investigated. The results from X-ray diffraction and scanning electron microscope indicated that the crystallinity of the synthesized zeolite is the most important factor to affect the CEC. When the synthesized zeolite with the highest CEC (275.5 meq/100 g) was used for the adsorption of Cr(VI) from aqueous solution, the maximum adsorption capacity for Cr(VI) was found to be 17.924 mg/g. The effects of pH, contact time and initial concentration on the adsorption of Cr(VI) were also investigated. The adsorption kinetics and isotherms can be well described by the pseudo-second-order model and Langmuir isotherm model, respectively.

Similar content being viewed by others
References
Zhang Y, Wu J, Wang Y, Miao Z, Si C, Shang X, Zhang N. Effect of hydrothermal dewatering on the physico-chemical structure and surface properties of Shengli lignite. Fuel, 2016, 164: 128–133
Kronbauer M A, Izquierdo M, Dai S, Waanders F B, Wagner N J, Mastalerz M, Hower J C, Oliveira ML S, Taffarel S, Bizani D, Silva L F O. Geochemistry of ultra-fine and nano-compounds in coal gasification ashes: A synoptic view. Science of the Total Environment, 2013, 456-457: 95–103
Minchener A J. Coal gasification for advanced power generation. Fuel, 2005, 84(17): 2222–2235
Blissett R S, Rowson N A. A review of the multi-component utilisation of coal fly ash. Fuel, 2012, 97: 1–23
Gustin M S, Ladwig K. An assessment of the significance of mercury release from coal fly ash. Journal of the Air & Waste Management Association, 2004, 54(3): 320–330
Choi S K, Lee S, Song Y K, Moon H S. Leaching characteristics of selected Korean fly ashes and its implications for the groundwater composition near the ash disposal mound. Fuel, 2002, 81(8): 1083–1090
Ahmaruzzaman M. A review on the utilization of fly ash. Progress in Energy and Combustion Science, 2010, 36(3): 327–363
Querol X, Moreno N, Umaña J C, Alastuey A, Hernández E, López-Soler A, Plana F. Synthesis of zeolites from coal fly ash: An overview. International Journal of Coal Geology, 2002, 50(1): 413–423
Yao Z T, Ji X S, Sarker P K, Tang J H, Ge L Q, Xia M S, Xi Y Q. A comprehensive review on the applications of coal fly ash. Earth-Science Reviews, 2015, 141: 105–121
Vyšvařil M, Bayer P. Immobilization of heavy metals in natural zeolite-blended cement pastes. Procedia Engineering, 2016, 151: 162–169
Shanableh A, Kharabsheh A. Stabilization of Cd, Ni a.d Pb in soil using natural zeolite. Journal of Hazardous Materials, 1996, 45(2-3): 207–217
Ali I, Asim M, Khan T A. Low cost adsorbents for the removal of organic pollutants from wastewater. Journal of Environmental Management, 2012, 113: 170–183
Kunecki P, Panek R, Wdowin M, Franus W. Synthesis of faujasite (FAU) and tschernichite (LTA) type zeolites as a potential direction of the development of lime Class C fly ash. International Journal of Mineral Processing, 2017, 166: 69–78
Wdowin M, Franus M, Panek R, Badura L, Franus W. The conversion technology of fly ash into zeolites. Clean Technologies and Environmental Policy, 2014, 16(6): 1217–1223
Querol X, Umaña J C, Plana F, Alastuey A, Lopez-Soler A, Medinaceli A, Valero A, Domingo M J, Garcia-Rojo E. Synthesis of zeolites from fly ash at pilot plant scale. Examples of potential applications. Fuel, 2001, 80(6): 857–865
Molina A, Poole C. A comparative study using two methods to produce zeolites from fly ash. Minerals Engineering, 2004, 17(2): 167–173
Bai J, Li W, Li B. Characterization of low-temperature coal ash behaviors at high temperatures under reducing atmosphere. Fuel, 2008, 87(4-5): 583–591
Huffman G P, Huggins F E, Dunmyre G R. Investigation of the high-temperature behaviour of coal ash in reducing and oxidizing atmospheres. Fuel, 1981, 60(7): 585–597
Zhang Y, Dong J, Guo F, Shao Z, Wu J. Zeolite synthesized from coal fly ash produced by a gasification process for Ni2+ removal from water. Minerals (Basel), 2018, 8(3): 116
Xia S, Song Z, Jeyakumar P, Shaheen S M, Rinklebe J, Ok Y S, Bolan N, Wang H. A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Critical Reviews in Environmental Science and Technology, 2019, 49(12): 1027–1078
Jobby R, Jha P, Yadav A K, Desai N. Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere, 2018, 207: 255–266
Fu F, Wang Q. Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management, 2011, 92(3): 407–418
Bo S, Ren W, Lei C, Xie Y, Cai Y, Wang S, Gao J, Ni Q, Yao J. Flexible and porous cellulose aerogels/zeolitic imidazolate framework (ZIF-8) hybrids for adsorption removal of Cr(IV) from water. Journal of Solid State Chemistry, 2018, 262: 135–141
Visa M, Chelaru A M. Hydrothermally modified fly ash for heavy metals and dyes removal in advanced wastewater treatment. Applied Surface Science, 2014, 303: 14–22
Koukouzas N, Vasilatos C, Itskos G, Mitsis I, Moutsatsou A. Removal of heavy metals from wastewater using CFB-coal fly ash zeolitic materials. Journal of Hazardous Materials, 2010, 173(1-3): 581–588
Ojha K, Pradhan N C, Samanta A N. Zeolite from fly ash synthesis and characterization. Bulletin of Materials Science, 2004, 27(6): 555–564
Zhang Y, Guo Y, Jiang Y, Guo F, Zhao X, Wu J. The mechanism of the ash fusion characteristics of gasification coke affected by SiO2/Al2O3 ratio and CaO content in blending coals. International Journal of Coal Preparation and Utilization. Online (Bergheim), 2019, doi:10.1080/19392699.2019.1675647
Shang X, Hou K, Wu J, Zhang Y, Liu J, Qi J. The influence of mineral matter on moisture adsorption property of Shengli lignite. Fuel, 2016, 182: 749–753
Zhang Y, Dong J, Guo F, Chen X, Wu J, Miao Z, Xiao L. Effects of the evolutions of coal properties during nitrogen and MTE drying processes on the spontaneous combustion behavior of Zhaotong lignite. Fuel, 2018, 232: 299–307
Li F, Liu Q, Li M, Fang Y. Understanding fly-ash formation during fluidized-bed gasification of high-silicon-aluminum coal based on its characteristics. Energy, 2018, 150: 142–152
Moreno N, Querol X, Andres J, Stanton K, Towler M, Nugteren H, Janssen-Jurkovicova M, Jones R. Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel, 2005, 84(11): 1351–1363
Rayalu S, Meshram S U, Hasan M Z. Highly crystalline faujasitic zeolites from fly ash. Journal of Hazardous Materials, 2000, 77(1-3): 123–131
Shigemoto N, Hayashi H, Miyaura K. Selective formation of Na-X zeolite from coal fly ash by fusion with sodium hydroxide prior to hydrothermal reaction. Journal of Materials Science, 1993, 28(17): 4781–4786
Kazemian H, Naghdali Z, Ghaffari Kashani T, Farhadi F. Conversion of high silicon fly ash to Na-P1 zeolite: Alkaline fusion followed by hydrothermal crystallization. Advanced Powder Technology, 2010, 21(3): 279–283
Liu Y, Yan C, Zhao J, Zhang Z, Wang H, Zhou S, Wu L. Synthesis of zeolite P1 from fly ash under solvent-free conditions for ammonium removal from water. Journal of Cleaner Production, 2018, 202: 11–22
Yusof A M, Malek N A. Removal of Cr(VI) and As(V) from aqueous solutions by HDTMA-modified zeolite Y. Journal of Hazardous Materials, 2009, 162(2-3): 1019–1024
Cotton F A, Wilkinson G. Advanced Inorganic Chemistry. 5th ed. New York: Wiley, 1998, 29–32
Barrera-Diaz C E, Lugo-Lugo V, Bilyeu B. A review of chemical, electrochemical a.d biological methods for aqueous Cr(VI) reduction. Journal of Hazardous Materials, 2012, 223-224: 1–12
Dhal B, Thatoi H N, Das N N, Pandey B D. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review. Journal of Hazardous Materials, 2013, 250-251: 272–291
Onundi Y B, Mamun A A, Khatib M F A, Ahmed Y M. Adsorption of copper, nickel a.d lead ions from synthetic semiconductor industrial wastewater by palm shell activated carbon. International Journal of Environmental Science and Technology, 2010, 7(4): 751–758
Rao M, Parwate A V, Bhole A G. Removal of Cr6+ and Ni2+ from aqueous solution using bagasse and fly ash. Waste Management (New York, N.Y.), 2002, 22(7): 821–830
Ho Y S, McKay G. Pseudo-second order model for sorption processes. Process Biochemistry, 1999, 34(5): 451–465
Kocaoba S, Orhan Y, Akyüz T. Kinetics and equilibrium studies of heavy metal ions removalby use of natural zeolite. Desalination, 2007, 214(1-3): 1–10
Abdel Salam O E, Reiad N A, ElShafei M M. A study of the removal characteristics of heavy metals from wastewater by low-cost adsorbents. Journal of Advanced Research, 2011, 2(4): 297–303
Sreenivas K M, Inarkar M B, Gokhale S V, Lele S S. Re-utilization of ash gourd (Benincasa hispida) peel waste for chromium (VI) biosorption: Equilibrium and column studies. Journal of Environmental Chemical Engineering, 2014, 2(1): 455–462
Gupta V K, Gupta M, Sharma S. Process development for the removal of lead and chromium from aqueous solutions using red mud-an aluminium industry waste. Water Research, 2001, 35(5): 1125–1134
Selvaraj K, Manonmani S, Pattabhi S. Removal of hexavalent chromium using distillery sludge. Bioresource Technology, 2003, 89(2): 207–211
Aydın Y A, Aksoy N D. Adsorption of chromium on chitosan: Optimization, kinetics a.d thermodynamics. Chemical Engineering Journal, 2009, 151(1-3): 188–194
McLellan J K, Rock C A. Pretreating landfill leachate with peat to remove metals. Water, Air, and S.il Pollution, 1988, 37(1): 203–215
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (No. 2017QNA25).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhou, L., Chen, L. et al. Synthesis of zeolite Na-P1 from coal fly ash produced by gasification and its application as adsorbent for removal of Cr(VI) from water. Front. Chem. Sci. Eng. 15, 518–527 (2021). https://doi.org/10.1007/s11705-020-1926-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-020-1926-9