Abstract
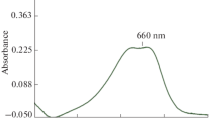
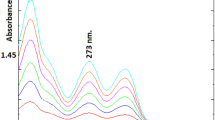
Based on the absorption property of a diazine that can be formed by reaction of glyoxal and 3-methyl-2-benzothiazolinone hydrazone (MBTH) in the Ultraviolet-visible (UV-vis) spectral region, a HPLC method was developed for the determination of glyoxal in acetaldehyde solution. Glyoxal was derivatised from MBTH and the derivatives (diazine) were analyzed by HPLC for identification and quantification. The determination was performed on a ZORBAX Eclipse XDB-C18 column (4.6 × 250 mm, 5 μm) at 35°C with an injection volume of 10 μL, using a mixture of acetonitrile-water solvent (99:5, v:v) as a mobile phase with a flow rate of 0.8 mL·min−1. The proper derivative reaction conditions were the temperature of 70°C, MBTH to carbonyl molar ratio of 12, and reaction time of 110 min. The glyoxal diazine was a yellow dye with a maximum molar absorptivity at 401 nm and its retention time was 5.2 min under optimal HPLC conditions. The standard curve for glyoxal had a strong linear relationship with a regression coefficient (R 2 = 0.999) in the range of 0.002–0.020 g·L−1. The analysis of glyoxal in an oxidising solution gave accurate results with a relative standard deviation (RSD) value of 0.55%. The average relative recovery was 102%. This efficient HPLC technique is also proposed for detecting other dicarbonyl compounds besides glyoxal.
Similar content being viewed by others
References
Nakajima K, Ohta K, Mostefaoui T A, Chai W, Utsukihara T, Horiuchi C A, Murakami M. Glyoxal sample preparation for highperformance liquid chromatographic detection of 2,4-dinitrophenylhydrazone derivative: Suppression of polymerization and mono-derivative formation by using methanol medium. Journal of Chromatography. A, 2007, 1161(1–2): 338–341
Ke B. Production status and application of glyoxal. Petrochemical, 1994, 23: 412–415 (in Chinese)
Howe B K, Hardy F R F, Clarke D A. GB Patent, 1272592A, 1972
Vodyankina O V, Koscheev S V, Yakushko V T, Salanov A N, Boronin A I, Kurina L N. Physicochemical investigation of the copper and silver catalysts of the ethylene glycol oxidation. Journal of Molecular Catalysis A: Chemical, 2008, 158(1): 381–387
Nede E. GB Patent, 3429929, 1969
Hotanahalli S S, Chandalia S B. Oxidation of acetaldehyde to glyoxal by nitric acid. J appl Chem Biotechnol, 1972, 22: 1243–1252
Analytical Chemistry Staff, Room of Department of Chemistry, Hangzhou University. Analytical Chemistry Manual (the second fascicule): Chemical Analysis. Beijing: Chemical Industry Press, 1996 (in Chinese)
Hu J, Zhang X, Wu M. Chemical analysis method of electric oxidation of glyoxal to glyoxalic acid. Journal of East China University of Science and Technology, 2001, 27: 34–37 (in Chinese)
Uchiyama S, Matsushima E, Tokunaga H, Otsubo Y, Ando M. Determination of orthophthalaldehyde in air using 2,4-dinitrophenylhydrazine-impregnated silica cartridge and high-performance liquid chromatography. Journal of Chromatography. A, 2006, 1116(1–2): 165–171
Strassnig S, Wenzl T, Lankmayr E P. Microwave-assisted derivatization of volatile carbonyl compounds with O-(2,3,4,5,6-pentafluorobenzyl) hydroxylamine. Journal of Chromatography. A, 2000, 891(2): 267–273
Cancho B, Ventura F, Galceran M T. Determination of aldehydes in drinking water using pentafluorobenzylhydroxylamine derivatization and solid-phase microextraction. Journal of Chromatography. A, 2001, 943(1): 1–13
Culleré L, Cacho J, Ferreira V. Analysis for wine C5-C8 aldehydes through the determination of their O-(2,3,4,5,6-pentafluorobenzyl) oximes formed directly in the solid phase extraction cartridge. Analytica Chimica Acta, 2004, 524(1–2): 201–206
Wang Q, O’Reilly J, Pawliszyn J. Determination of low-molecular mass aldehydes by automated headspace solid-phase microextraction with in-fibre derivatisation. Journal of Chromatography. A, 2005, 1071(1–2): 147–154
Pacolay B D, Ham J E, Wells J R. Use of solid-phase microextraction to detect and quantify gas-phase dicarbonyls in indoor environments. Journal of Chromatography. A, 2006, 1131(1–2): 275–280
Svensson S, Larstad M, Broo K, Anna-Carin O. Determination of aldehydes in human breath by on-fibre derivatization, solid-phase microextraction and GC-MS. Journal of Chromatography. A, 2007, 860: 86–91
Beránek J, Kubátová A. Evaluation of solid-phase microextraction methods for determination of trace concentration aldehydes in aqueous solution. Journal of Chromatography. A, 2008, 1209(1–): 44–54
Smith D F, Kleindienst T E, Hudgens E E. Improved high-performance liquid chromatographic method for artifact-free measurements of aldehydes in the presence of ozone using 2,4-dinitrophenylhydrazine. Journal of Chromatography. A, 1989, 483: 431–436
Druzik C M, Grosjean D, Neste A V, Parmar S S. Sampling of atmospheric carbonyls with small DNPH-coating C18 cartridges and liquid chromatography analysis with diode array detection. International Journal of Environmental Analytical Chemistry, 1990, 38(4): 495–512
Grosjean E, Grosjean D. Liquid chromatographic analysis of C1–C10 carbonyls. International Journal of Environmental Analytical Chemistry, 1995, 61(1): 47–64
METHOD. 8315A. Determination of Carbonyl Compounds by High Performance Liquid Chromatography (HPLC), U.S. Environmental Protection Agency, 1996
Ho S S, Yu J Z. Determination of airborne carbonyls: comparison of a thermal desorption/GC method with the standard DNPH/HPLC method. Environmental Science & Technology, 2004, 38(3): 862–870
Zhu Y, Cui Q, Wang H. Determination of glyoxal and glyoxalic acid in aldehyde solution by high performance liquid chromatography. J Chromatogr, 2010, 28(1): 59–63 (in Chinese)
Neumann F W. Spectrophotometric determination of glyoxal with 3-methyl-2-benzothiazolinone hydrazone. Analytical Chemistry, 1969, 41(14): 2077–2078
Igawa M, Munger J W, Hoffmann M R. Analysis of aldehydes in cloud- and fogwater samples by HPLC with a postcolumn reaction detector. Environmental Science & Technology, 1989, 23(5): 556–561
Zurek G, Karst U. Microplate photometric determination of aldehydes in disinfectant solutions. Analytica Chimica Acta, 1997, 351(1–3): 247–257
General Administration of Quality Supervision Inspection and Quarantine of the People’s Republic of China (AQSIQ). GB/T 6324.5-2008
Zhu Y, Jiang Y, Cui Q, Wang H. Study on oxidation of acetaldehyde to glyoxal by nitric acid chemical reaction engineering and technology. Chemical Reaction Engineering and Technology, 2009, 25: 175–179 (in Chinese)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhu, Y., Yao, X., Chen, S. et al. HPLC determination of glyoxal in aldehyde solution with 3-methyl-2-benzothiazolinone hydrazone. Front. Chem. Sci. Eng. 5, 117–121 (2011). https://doi.org/10.1007/s11705-010-0535-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-010-0535-4