Abstract
Background
Roux-en-Y gastric bypass (RYGB) is an effective surgical treatment for type 2 diabetes mellitus (T2DM). The present study aimed to investigate the effects of RYGB on glucose homeostasis, lipid metabolism, and intestinal morphological adaption, as well as hepatic and intestinal gluconeogenesis.
Methods
Twenty adult male T2DM rats induced by high-fat diet and low dose of streptozotocin were randomly divided into sham and RYGB groups. The parameters of body weight, food intake, glucose tolerance, insulin sensitivity, and serum lipid profiles were assessed to evaluate metabolic changes. Intestinal sections were stained with hematoxylin and eosin (H&E) for light microscopy examination. The messenger RNA (mRNA) and protein expression levels of key regulatory enzymes of gluconeogenesis [phosphoenolpyruvate carboxykinase (PEPCK), glucose-6-phosphatase (G6Pase)] were determined through reverse-transcription PCR (RT-PCR) and Western blotting, respectively.
Results
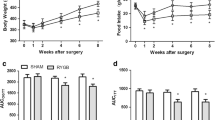
RYGB induced significant improvements in glucose tolerance and insulin sensitivity, along with weight loss and decreased food intake. RYGB also decreased serum triglyceride (TG) and free fatty acid (FFA) levels. The jejunum and ileum exhibited a marked increase in the length and number of intestinal villi after RYGB. The RYGB group exhibited downregulated mRNA and protein expression levels of PEPCK and G6Pase in the liver and upregulated expression of these enzymes in the jejunum and ileum tissues.
Conclusions
RYGB ameliorates glucose and lipid metabolism accompanied by weight loss and calorie restriction. The small intestine shows hyperplasia and hypertrophy after RYGB. Meanwhile, our study demonstrated that the reduced hepatic gluconeogenesis and increased intestinal gluconeogenesis may contribute to improved glucose homeostasis after RYGB.




Similar content being viewed by others
References
Kharroubi AT, Darwish HM. Diabetes mellitus: the epidemic of the century. World J Diabetes. 2015;6(6):850–67.
Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934.
Muller-Stich BP, Senft JD, Warschkow R, et al. Surgical versus medical treatment of type 2 diabetes mellitus in nonseverely obese patients: a systematic review and meta-analysis. Ann Surg. 2015;261(3):421–9.
Angrisani L, Santonicola A, Iovino P, et al. Bariatric Surgery Worldwide 2013. Obes Surg. 2015;25(10):1822–32.
Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2(2):152–64.
Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341(6144):406–10.
Mumphrey MB, Patterson LM, Zheng H, et al. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil. 2013;25(1):e70–9.
Mithieux G. Nutrient control of energy homeostasis via gut-brain neural circuits. Neuroendocrinology. 2014;100(2-3):89–94.
Hayes MT, Foo J, Besic V, et al. Is intestinal gluconeogenesis a key factor in the early changes in glucose homeostasis following gastric bypass? Obes Surg. 2011;21(6):759–62.
SAGES Guidelines Committee. SAGES guideline for clinical application of laparoscopic bariatric surgery. Surg Obes Relat Dis. 2009;5(3):387-405.
Serrot FJ, Dorman RB, Miller CJ, et al. Comparative effectiveness of bariatric surgery and nonsurgical therapy in adults with type 2 diabetes mellitus and body mass index <35 kg/m2. Surgery. 2011;150(4):684–91.
Cohen RV, Pinheiro JC, Schiavon CA, et al. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35(7):1420–8.
Boza C, Munoz R, Salinas J, et al. Safety and efficacy of Roux-en-Y gastric bypass to treat type 2 diabetes mellitus in non-severely obese patients. Obes Surg. 2011;21(9):1330–6.
Ikramuddin S, Billington CJ, Lee WJ, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol. 2015;3(6):413–22.
Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149(7):716–26.
Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149(7):707–15.
Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–20.
Jurowich CF, Rikkala PR, Thalheimer A, et al. Duodenal-jejunal bypass improves glycemia and decreases SGLT1-mediated glucose absorption in rats with streptozotocin-induced type 2 diabetes. Ann Surg. 2013;258(1):89–97.
Li M, Li H, Zhou Z, et al. Duodenal-jejunal bypass surgery ameliorates glucose homeostasis and reduces endoplasmic reticulum stress in the liver tissue in a diabetic rat model. Obes Surg. 2015. doi:10.1007/s11695-015-1816-2
Jurowich CF, Otto C, Rikkala PR, et al. Ileal interposition in rats with experimental type 2 like diabetes improves glycemic control independently of glucose absorption. J Diabetes Res. 2015;2015:490365.
Xu B, Yan X, Shao Y, et al. A comparative study of the effect of gastric bypass, sleeve gastrectomy, and duodenal-jejunal bypass on type-2 diabetes in non-obese rats. Obes Surg. 2015;25(10):1966–75.
Bhutta HY, Rajpal N, White W, et al. Effect of Roux-en-Y gastric bypass surgery on bile acid metabolism in normal and obese diabetic rats. PLoS One. 2015;10(3):e0122273.
Chambers AP, Jessen L, Ryan KK, et al. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology. 2011;141(3):950–8.
Bartoli E, Fra GP, Carnevale Schianca GP. The oral glucose tolerance test (OGTT) revisited. Eur J Intern Med. 2011;22(1):8–12.
Han H, Hu C, Wang L, et al. Duodenal-jejunal bypass surgery suppresses hepatic de novo lipogenesis and alleviates liver fat accumulation in a diabetic rat model. Obes Surg. 2014;24(12):2152–60.
Sun Y, Li W, Hou X, et al. Triglycerides and ratio of triglycerides to high-density lipoprotein cholesterol are better than liver enzymes to identify insulin resistance in urban middle-aged and older non-obese Chinese without diabetes. Chin Med J. 2014;127(10):1858–62.
Spak E, Bjorklund P, Helander HF, et al. Changes in the mucosa of the Roux-limb after gastric bypass surgery. Histopathology. 2010;57(5):680–8.
le Roux CW, Borg C, Wallis K, et al. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg. 2010;252(1):50–6.
Taqi E, Wallace LE, de Heuvel E, et al. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg. 2010;45(5):987–95.
Sharabi K, Tavares CD, Rines AK, et al. Molecular pathophysiology of hepatic glucose production. Mol Aspects Med. 2015; 46:21–33.
Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2003;285(4):E685–92.
Troy S, Soty M, Ribeiro L, et al. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab. 2008;8(3):201–11.
Soty M, Penhoat A, Amigo-Correig M, et al. A gut-brain neural circuit controlled by intestinal gluconeogenesis is crucial in metabolic health. Mol Metab. 2015;4(2):106–17.
Kim M, Son YG, Kang YN, et al. Changes in glucose transporters, gluconeogenesis, and circadian clock after duodenal-jejunal bypass surgery. Obes Surg. 2015;25(4):635–41.
Sun D, Wang K, Yan Z, et al. Duodenal-jejunal bypass surgery up-regulates the expression of the hepatic insulin signaling proteins and the key regulatory enzymes of intestinal gluconeogenesis in diabetic Goto-Kakizaki rats. Obes Surg. 2013;23(11):1734–42.
Acknowledgments
This work was supported by the Key Project of Shanghai Health and Family Planning Commission (Grant No. 201440026).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
All authors declare that we have no conflict of interest.
Statement of Informed Consent
Informed consent does not apply to this study.
Statement of Human and Animal Rights
All applicable institutional and national guidelines for the care and use of animals were followed.
Additional information
Yong Yan and Zhou Zhou contributed equally to this work. Cheng Hu and Xueli Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yan, Y., Zhou, Z., Kong, F. et al. Roux-en-Y Gastric Bypass Surgery Suppresses Hepatic Gluconeogenesis and Increases Intestinal Gluconeogenesis in a T2DM Rat Model. OBES SURG 26, 2683–2690 (2016). https://doi.org/10.1007/s11695-016-2157-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-016-2157-5