Abstract
Background
Primary Obesity Surgery Endolumenal (POSE) is a novel bariatric endoscopic procedure that has been shown to reduce weight safely through 12 months. The study investigated potential mechanisms of weight loss following POSE.
Methods
Patients with class I–II obesity received transmural plications in the gastric fundus and distal gastric body. Patients were evaluated at baseline and at 2- and 6-month follow-up with gastric-emptying (GE) scintigraphy, a validated test of intake capacity (kcal) and plasma glucose homeostasis hormones/gastrointestinal peptides. Weight was recorded through 15 months. Mean data and 95 % CIs are reported. Regression modeling assessed variables that influenced total weight loss (%TWL) and excess weight loss (%EWL).
Results
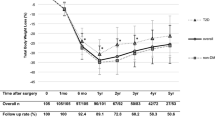
POSE was performed on 18 patients (14 F/4 M); mean age 39 years (34–44), body mass index (BMI, kg/m2) 36 (95 % CI, 35; 37). At 15 months (n = 15), mean TWL was 19.1 ± 6.6 % (15.5; 22.8) and EWL was 63.7 ± 25.1 % (49.8; 77.6). At 2 and 6 months (n = 18), intake capacity decreased significantly from 901 (685; 1117) to 473 (345; 600) and 574 kcal (418; 730), respectively (p < 0.001). At 2 months, GE was delayed but returned to baseline levels at 6 months (n = 18). Glucose/insulin ratio improved (p < 0.05). Postprandial decrease in ghrelin was enhanced (p = 0.03) as well as postprandial increase in PYY (p = 0.001). The best model for EWL prediction 15 months after POSE (R 2: 66 %, p = 0.006) included pre-POSE BMI, post-POSE GE, and postprandial PYY increase.
Conclusions
The POSE procedure was followed by significant sustained weight loss and improved glucose homeostasis and satiation peptide responses. Weight loss following POSE may be mediated through changes in gastrointestinal neuro-endocrine physiology.






Similar content being viewed by others
References
Colquitt JL, Picot J, Loveman E, et al. Surgery for obesity. Cochrane Database Syst Rev. 2009;2:Cd003641.
Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014;m24(3):437–55.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Buchwald H, Estok R, Fahrbach K, et al. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142(4):621–32.
Chang SH, Stoll CR, Song J, et al. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014;149(3):275–87.
Martin M, Beekley A, Kjorstad R, et al. Socioeconomic disparities in eligibility and access to bariatric surgery: a national population-based analysis. Surg Obes Relat Dis. 2010;6(1):8–15.
Espinos JC, Turro R, Mata A, et al. Early experience with the Incisionless Operating Platform (IOP) for the treatment of obesity: the Primary Obesity Surgery Endolumenal (POSE) procedure. Obes Surg. 2013;23(9):1375–83.
Lopez-Nava G, Bautistia-Castaño I, Jiminez A, et al. The Primary Obesity Surgery Endolumenal (POSE) procedure: one-year patient weight loss and safety outcomes. Surg Obes Relat Dis. 2014, in press.
Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol. 2004;286(1):G7–13.
Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014;15(6):367–78.
Delgado-Aros S, Cremonini F, Castillo JE, et al. Independent influences of body mass and gastric volumes on satiation in humans. Gastroenterology. 2004;126:432–40.
Vazquez Roque MI, Camilleri M, Stephens DA, et al. Gastric sensorimotor functions and hormone profile in normal weight, overweight, and obese people. Gastroenterol. 2006;131:1717–24.
Moragas G, Azpirov F, Pavia J, et al. Relations among intragastric pressure, postcibal perception, and gastric emptying. Am J Physiol. 1993;246(6 Part 1):G1112–7.
Gras-Miralles B, Haya JR, Moros JM, et al. Caloric intake capacity as measured by a standard nutrient drink test helps to predict weight loss after bariatric surgery. Obes Surg. 2014;24(12):2138–44.
Puigvehi M, Gras-Miralles B, Torra S, et al. Comparison of ad-libitum energy intake and the neuro-endocrine postprandial response as measured during a buffet-type meal and a standardized nutrient drink test. Gastroenterology 2012; 142:S-56.
Torra S, Ilzarbe L, Malagelada JR, et al. Meal size can be decreased in obese subjects through pharmacological acceleration of gastric emptying (The OBERYTH trial). Int J Obes (Lond). 2011;35(6):829–37.
Meyer-Gerspach AC, Wölnerhanssen B, Beglinger B, et al. Gastric and intestinal satiation in obese and normal weight healthy people. Physiol Behav. 2014;129:265–71.
Camilleri M, Colemont LJ, Phillips SF, et al. Human gastric emptying and colonic filling of solids characterized by a method. Am J Physiol. 1989;257(2 Part 1):G284–90.
Ginsberg GG, Chand B, Cote GA, et al. A pathway to endoscopic bariatric therapies. Gastrointest Endosc. 2011;74(5):943–53.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56.
Wilson PW, Kannel WB, Silbershatz H, et al. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159(10):1104–9.
Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–61.
Cummings DE, Purnell JQ, Frayo RS, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–9.
Tschop M, Weyer C, Tataranni PA, et al. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–9.
Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623–30.
Morinigo R, Vidal J, Lacy AM, et al. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247(2):270–5.
Adrian TE, Ferri GL, Bacarese-Hamilton AJ, et al. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–7.
Dockray GJ. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol. 2014;592:2927–41.
Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–7.
Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349(10):941–8.
le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246(5):780–5.
Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring). 2006;14(9):1553–61.
Lakhan SE, Kirchgessner A. Neuroinflammation in inflammatory bowel disease. J Neuroinflamm. 2010;7:37.
Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9.
Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–8.
Tack J, Demedts I, Dehondt G, et al. Clinical and pathophysiological characteristics of acute-onset functional dyspepsia. Gastroenterol. 2002;122:1738–47.
Mearin F, Perez-Oliveras M, Perello A, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterol. 2005;129:98–104.
Acknowledgments
We wish to thank Mónica Isart Rueda, director of Laboratory Services at Teknon; Javier Pavia for the assistance with gastric emptying analysis; and Maribel Sánchez for the nutritional support with patients during the study.
Conflict of Interest
Manuscript development was financially supported by USGI Medical, Inc., USA. A. Bronstone is a Scientific Research Writer and J. N. Buchwald is the Director and Chief Scientific Research Writer at Medwrite LLC, a CRO under contract with USGI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Espinós, J.C., Turró, R., Moragas, G. et al. Gastrointestinal Physiological Changes and Their Relationship to Weight Loss Following the POSE Procedure. OBES SURG 26, 1081–1089 (2016). https://doi.org/10.1007/s11695-015-1863-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1863-8