Abstract
Background
Obese patients have a higher risk of venous thromboembolism when immobilized due to surgery. The objective of this study was to assess anti-factor Xa activity in adolescent bariatric surgical patients receiving prophylactic enoxaparin.
Methods
Four morbidly obese adolescents undergoing laparoscopic sleeve gastrectomy were enrolled. Enoxaparin was administered (40 mg subcutaneous (SC) if BMI ≤50 kg/m2 or 60 mg SC if BMI >50 kg/m2) for prevention of venous thromboembolism every 12 h starting after induction of anesthesia until discharge. Plasma anti-factor Xa activity was assessed over 12 h after the first dose and used as a surrogate marker for enoxaparin levels. Non-compartmental analysis of anti-factor Xa activity levels was performed and compared with previously published studies.
Results
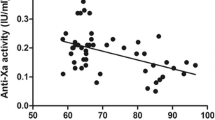
Patients recruited were 16 to 18 years of age with a mean BMI of 52.6 ± 5.8 kg/m2 (>99th BMI percentile). Peak anti-factor Xa activity ranged from 0.20 to 0.23 IU/mL in our study population, compared to 0.38 to 0.53 IU/mL in the cited lean comparator groups.
Conclusions
Our current dosing practice of 40 mg SC for individuals with a BMI ≤50 kg/m2 and 60 mg for individuals with a BMI ≥50 kg/m2 resulted in anti-factor Xa activity that was sufficient for adequate thromboprophylaxis in adolescent bariatric surgical patients. Our data also demonstrates lower drug exposures in the obese when compared to lean patients. Therefore, randomized controlled efficacy and safety studies are urgently needed to guide the use of low-molecular-weight heparins in the pediatric and adolescent obese population.


Similar content being viewed by others
References
Castro PS, Oliveira FL. Prevention of atherosclerosis and drug treatment of high-risk lipid abnormalities in children and adolescents. J Pediatr (Rio J). 2009;85(1):6–14.
Borch KH et al. Joint effects of obesity and body height on the risk of venous thromboembolism: the Tromso Study. Arterioscler Thromb Vasc Biol. 2011;31(6):1439–44.
American College of Chest Physicians. Correction to dosage in: parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2013;144(2):721. doi:10.1378/chest.13-1396
Rowan BO et al. Anti-Xa levels in bariatric surgery patients receiving prophylactic enoxaparin. Obes Surg. 2008;18(2):162–6.
Azizi M et al. Comparison of biological activities of two low molecular weight heparins in 10 healthy volunteers. Br J Clin Pharmacol. 1995;40(6):577–84.
Frydman AM et al. The antithrombotic activity and pharmacokinetics of enoxaparin, a low molecular weight heparin, in humans given single subcutaneous doses of 20 to 80 mg. J Clin Pharmacol. 1988;28(7):609–18.
Funk DM. Coagulation assays and anticoagulant monitoring. Hematol Am Soc Hematol Educ Prog. 2012;2012:460–5.
Raffini L et al. Thromboprophylaxis in a pediatric hospital: a patient-safety and quality-improvement initiative. Pediatrics. 2011;127(5):e1326–32.
Rondina MT et al. Weight-based dosing of enoxaparin for VTE prophylaxis in morbidly obese, medically-Ill patients. Thromb Res. 2010;125(3):220–3.
Rocha AT et al. Risk of venous thromboembolism and efficacy of thromboprophylaxis in hospitalized obese medical patients and in obese patients undergoing bariatric surgery. Obes Surg. 2006;16(12):1645–55.
Lewis TV et al. Increased enoxaparin dosing is required for obese children. Pediatrics. 2011;127(3):e787–90.
Maxwell BG et al. Perioperative management of the morbidly obese adolescent with heart failure undergoing bariatric surgery. Paediatr Anaesth. 2012;22(5):476–82.
Monagle P et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e737S–801.
Massicotte P et al. An open-label randomized controlled trial of low molecular weight heparin for the prevention of central venous line-related thrombotic complications in children: the PROTEKT trial. Thromb Res. 2003;109(2–3):101–8.
Traish AM, Galoosian A. Androgens modulate endothelial function and endothelial progenitor cells in erectile physiology. Korean J Urol. 2013;54(11):721–31.
Borkgren-Okonek MJ et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4(5):625–31.
Deal EN et al. Evaluation of therapeutic anticoagulation with enoxaparin and associated anti-Xa monitoring in patients with morbid obesity: a case series. J Thromb Thrombolysis. 2011;32(2):188–94.
Ludwig KP et al. Implementation of an enoxaparin protocol for venous thromboembolism prophylaxis in obese surgical intensive care unit patients. Ann Pharmacother. 2011;45(11):1356–62.
Freeman A et al. Prospective comparison of three enoxaparin dosing regimens to achieve target anti-factor Xa levels in hospitalized, medically ill patients with extreme obesity. Am J Hematol. 2012;87(7):740–3.
Acknowledgments
We acknowledge Elaine Williams RN, MSN, for help with blood sample collection and support with study coordination. We also thank Dr. Nicole Verdun for help with planning study and assessing results.
Conflict of Interest
Dr. Alvina Mushtaq has nothing to disclose.
Dr. Janelle D. Vaughns has nothing to disclose.
Dr. Victoria C. Ziesenitz has nothing to disclose.
Dr. Evan P. Nadler has nothing to disclose.
Dr. John N. van den Anker has nothing to disclose.
Statement of Informed Consent
Informed consent and assent were obtained from all individual participants included in the study.
Statement of Human and Animal Rights
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee, with the 1964 Helsinki declaration and its later amendments, and national laws for data protection. Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Alvina Mushtaq and Janelle D. Vaughns contributed equally to this work.
Rights and permissions
About this article
Cite this article
Mushtaq, A., Vaughns, J.D., Ziesenitz, V.C. et al. Use of Enoxaparin in Obese Adolescents During Bariatric Surgery—a Pilot Study. OBES SURG 25, 1869–1874 (2015). https://doi.org/10.1007/s11695-015-1630-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1630-x