Abstract
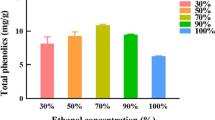
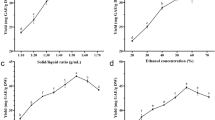
In recent years, propolis has generated significant interest in traditional medicine. Its extraction is a necessary process that requires optimal conditions that strongly influence extraction yield, chemical composition, and biological activity. The current study sought to evaluate the possible effects of pulverized propolis extract (PP) extracted from water-based Turkish propolis (WBTP) extract at low temperatures (+ 4, -80, and - 196 °C) on extraction yield, total phenolic content (TPC), total flavonoid content (TFC), and antioxidant, antibacterial, enzyme inhibition, and DNA protective activities. The yield with the highest extraction efficiency was PP-196 (38.86%), while the PP-80 extract had the highest content of TPC (13.99 ± 0.03 mg GAE/g) and TFC (3.38 ± 0.01 mg QE/g). The HPLC-MS/MS of PP-80 extract showed the highest levels of chrysin (8708.28 µg/mL), o-coumaric acid (2939.24 µg/mL), caffeic acid (1734.35 µg/mL), kaempferol (909.61 µg/mL), p-coumaric acid (900.18 µg/mL), tamarixetin (850.10 µg/mL), morin (648.46 µg/mL), and trans-ferulic acid (619.26 µg/mL). 1 H NMR spectral analysis confirmed that the PP-80 and PP + 4 extracts exhibited high signal intensities for galangin, chrysin, ramnocitrin, genkwanin, tectochrysin, and 3-metoxycamherol. The WBTP extracts were found to be effective free radical scavengers (DPPH˙, ABTS˙+, O2˙−), and the PP-80 extract had a high adequate reducing power (p ˂0.05). PP-80 and PP-196 extracts had more significant inhibitory effects on acetylcholinesterase, butyrylcholinesterase, α-glucosidase, lipase, and tyrosinase than the standards (p ˂0.05). PP-196 extract was active against P. aeruginosa, S. enterica, E. faecalis, B. cereus, and L. monocytogenes. All extracts presented considerable DNA protection activity, ranging from 73 to 85%. These findings demonstrate that PP-80 and PP-196 extracts are rich in phenolic components and possess powerful biochemical properties, supporting their proven use as dietary supplements for human health.







Similar content being viewed by others
References
V.C. Toreti et al., Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evidence-based complementary and alternative medicine, 2013. 2013
A. Salatino, M.L.F. Salatino, Scientific note: often quoted, but not factual data about propolis composition. Apidologie 52(2), 312–314 (2021)
M. Surek et al., Chemical composition, cytotoxicity, and antibacterial activity of propolis from africanized honeybees and three different Meliponini species. J. Ethnopharmacol. 269, 113662 (2021)
L. Moreira et al., Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 46(11), 3482–3485 (2008)
M. Viuda-Martos et al., Functional properties of honey, propolis, and royal jelly. J. Food Sci. 73(9), R117–R124 (2008)
L. Zhao et al., Brazilian green propolis improves antioxidant function in patients with type 2 diabetes mellitus. Int. J. Environ. Res. Public Health 13(5), 498 (2016)
E. Guzelmeric et al., Comparison of antioxidant and anti-inflammatory activity profiles of various chemically characterized turkish propolis sub-types: which propolis type is a promising source for pharmaceutical product development? Journal of Pharmaceutical and Biomedical Analysis, 2021: p. 114196
V. Bankova, B. Trusheva, M. Popova, Propolis extraction methods: a review. Journal of Apicultural Research, 2021: p. 1–10
M. Oroian, F. Ursachi, F. Dranca, Influence of ultrasonic amplitude, temperature, time and solvent concentration on bioactive compounds extraction from propolis. Ultrason. Sonochem. 64, 105021 (2020)
E. Golmakani et al., Phenolic and flavonoid content and antioxidants capacity of pressurized liquid extraction and perculation method from roots of Scutellaria pinnatifida A. Hamilt. Subsp alpina (Bornm) Rech. F. J. Supercrit. Fluids 95, 318–324 (2014)
Y. Zhang et al., Simultaneous determination of 20 phenolic Compounds in Propolis by HPLC-UV and HPLC-MS/MS. Journal of Food Composition and Analysis, 2022: p. 104877
T. Ozen et al., An investigation of chemical content, enzyme inhibitory propert, antioxidant and antibacterial activity of Aristolochia bodamae Dingler (develiotu)(Aristolochiaceae) root extracts from Samsun, Turkey. Flavour Fragr. J. 35(3), 270–283 (2020)
P. Prieto, M. Pineda, M. Aguilar, Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 269(2), 337–341 (1999)
M. Oyaizu, Studies on products of browning reaction. Japanese J. Nutr. dietetics 44(6), 307–315 (1986)
M.S. Blois, Antioxidant determinations by the use of a stable free radical. Nature 181(4617), 1199 (1958)
M. Noshad et al., Utilization of Plantago major seed mucilage containing Citrus limon essential oil as an edible coating to improve shelf-life of buffalo meat under refrigeration conditions. Food Sci. Nutr. 9(3), 1625–1639 (2021)
R. Re et al., Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 26(9–10), 1231–1237 (1999)
T.C. Dinis, V.M. Madeira, L.M. Almeida, Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 315(1), 161–169 (1994)
M. Nishikimi, N.A. Rao, K. Yagi, The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 46(2), 849–854 (1972)
X.-W. Yang et al., Phenolics from Bidens bipinnata and their amylase inhibitory properties. Fitoterapia 83(7), 1169–1175 (2012)
R. Trentin et al., Exploring Ulva australis Areschoug for possible biotechnological applications: in vitro antioxidant and enzymatic inhibitory properties, and fatty acids contents. Algal Res. 50, 101980 (2020)
N. Bibi Sadeer et al., Towards the Pharmacological Validation and Phytochemical Profiling of the Decoction and Maceration of Bruguiera gymnorhiza (L.) Lam.—A Traditionally Used Medicinal Halophyte. Molecules, 2022. 27(6): p. 2000
B. Mayur et al., Antioxidant and α-glucosidase inhibitory properties of Carpesium abrotanoides L. J. Med. Plants Res. 4(15), 1547–1553 (2010)
J. Chanda et al., UPLC-QTOF‐MS analysis of a carbonic anhydrase‐inhibiting extract and fractions of Luffa acutangula (L.) Roxb (ridge gourd). Phytochem. Anal. 30(2), 148–155 (2019)
L. Zhang et al., Inhibition of urease by bismuth (III): implications for the mechanism of action of bismuth drugs. Biometals 19(5), 503–511 (2006)
M. Yeganegi et al., Equisetum telmateia extracts: Chemical compositions, antioxidant activity and antimicrobial effect on the growth of some pathogenic strain causing poisoning and infection. Microb. Pathog. 116, 62–67 (2018)
H. Tanavar et al., Investigation of the Chemical Properties of Mentha pulegium Essential oil and its Application in Ocimum basilicum seed Mucilage Edible Coating for Extending the Quality and Shelf life of veal Stored in Refrigerator (4° C). Food Science & Nutrition, 2021. 9(10): pp. 5600–5615
A. Baiseitova et al., Phytochemical analysis of aerial part of Ikonnikovia kaufmanniana and their protection of DNA damage. Natural product research, 2019: p. 1–4
A. Russo et al., Free radical scavenging capacity and protective effect of Bacopa monniera L. on DNA damage. Phytotherapy Research: An International Journal devoted to pharmacological and toxicological evaluation of natural product derivatives, 2003. 17(8): p. 870–875
K. Sevgi, B. Tepe, C. Sarikurkcu, Antioxidant and DNA damage protection potentials of selected phenolic acids. Food Chem. Toxicol. 77, 12–21 (2015)
B. Tepe et al., Determination of chemical profile, antioxidant, DNA damage protection and antiamoebic activities of Teucrium polium and Stachys iberica. Fitoterapia 82(2), 237–246 (2011)
K. Pobiega et al., Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. J. Food Sci. Technology-Mysore 56(12), 5386–5395 (2019)
R.K. Kishore et al., Tualang honey has higher phenolic content and greater radical scavenging activity compared with other honey sources. Nutr. Res. 31(4), 322–325 (2011)
A. Rebiai et al., Fatty acid composition of algerian Propolis. J. Fundamental Appl. Sci. 9(3), 1656–1671 (2017)
M.L. Belfar et al., Evaluation of antioxidant capacity of Propolis Collected in various areas of Algeria using Electrochemical techniques. Int. J. Electrochem. Sci. 10(11), 9641–9651 (2015)
L.S. Olegario et al., Chemical characterization of four brazilian brown propolis: an insight in tracking of its geographical location of production and quality control. Food Res. Int. 123, 481–502 (2019)
B. Fumic, M. Jug, M.Z. Koncic, Multi-response optimization of Ultrasound-assisted extraction of Bioactive Components from Medicago sativa L. Croat. Chem. Acta 90(3), 481–491 (2017)
A.N. Tamfu et al., Antibiofilm and anti-quorum sensing potential of cycloartane-type triterpene acids from cameroonian grassland propolis: phenolic profile and antioxidant activity of crude extract. Molecules 27(15), 4872 (2022)
M. Yaghoubi, G. Gh, R. Satari, Antimicrobial activity of iranian propolis and its chemical composition. DARU J. Pharm. Sci. 15(1), 45–48 (2007)
V. Lagouri, D. Prasianaki, F. Krysta, Antioxidant properties and phenolic composition of greek propolis extracts. Int. J. Food Prop. 17(3), 511–522 (2014)
U. Siripatrawan, W. Vitchayakitti, R. Sanguandeekul, Antioxidant and antimicrobial properties of T hai propolis extracted using ethanol aqueous solution. Int. J. Food Sci. Technol. 48(1), 22–27 (2013)
N. Sha et al., Simultaneous quantification of eight major bioactive phenolic compounds in chinese propolis by high-performance liquid chromatography. Nat. Prod. Commun. 4(6), 1934578X0900400615 (2009)
J.K.S. Andrade et al., Evaluation of bioactive compounds, phytochemicals profile and antioxidant potential of the aqueous and ethanolic extracts of some traditional fruit tree leaves used in brazilian folk medicine. Food Res. Int. 143, 110282 (2021)
T. Ozdal et al., Investigation of antioxidant capacity, bioaccessibility and LC-MS/MS phenolic profile of turkish propolis. Food Res. Int. 122, 528–536 (2019)
J.S. Bonvehí, F.V. Coll, Phenolic composition of propolis from China and from South America. Z. für Naturforschung c 49(11–12), 712–718 (1994)
A. Li et al., Preparative separation of polyphenols from water-soluble fraction of chinese propolis using macroporous absorptive resin coupled with preparative high performance liquid chromatography. J. Chromatogr. B 1012, 42–49 (2016)
D. Bertelli et al., 1H-NMR simultaneous identification of Health‐Relevant Compounds in Propolis extracts. Phytochem. Anal. 23(3), 260–266 (2012)
E.T.D. Almeida et al., Chemical and microbiological characterization of tinctures and microcapsules loaded with brazilian red propolis extract. J. Pharm. Anal. 7(5), 280–287 (2017)
F.B. da Cruz et al., Antioxidant activity of Apis Mellifera Bee Propolis: a review. Journal of Natural Products Discovery, 2022. 1(1)
S. Boulechfar et al., Anticholinesterase, anti-α-glucosidase, antioxidant and antimicrobial effects of four algerian propolis. J. Food Meas. Charact. 16(1), 793–803 (2022)
I.G. Munteanu, C. Apetrei, Analytical methods used in determining antioxidant activity: a review. Int. J. Mol. Sci. 22(7), 3380 (2021)
E.E. Çelik, V. Gökmen, Interactions between free and bound antioxidants under different conditions in food systems. Crit. Rev. Food Sci. Nutr. 62(21), 5766–5782 (2022)
B. Mahdi-Pour et al., Antioxidant activity of methanol extracts of different parts of Lantana camara. Asian Pac. J. Trop. Biomed. 2(12), 960–965 (2012)
S. El-Guendouz et al., Anti‐acetylcholinesterase, antidiabetic, anti‐inflammatory, antityrosinase and antixanthine oxidase activities of moroccan propolis. Int. J. Food Sci. Technol. 51(8), 1762–1773 (2016)
P. Celso Pardi, G.A.A.d. Santos, Relationships Between Treatment and Clinical Evaluations, in Pharmacological Treatment of Alzheimer’s Disease (Springer, 2022), pp. 175–198
H.R. El-Seedi et al., Honeybee products: an updated review of neurological actions. Trends Food Sci. Technol. 101, 17–27 (2020)
S. Boulechfar et al., Anticholinesterase, anti-α-glucosidase, antioxidant and antimicrobial effects of four algerian propolis. Journal of Food Measurement and Characterization, 2021: p. 1–11
B. Vongsak et al., In vitro alpha glucosidase inhibition and free-radical scavenging activity of propolis from Thai stingless bees in mangosteen orchard. Revista Brasileira de Farmacognosia 25(5), 445–450 (2015)
N.P. Möller, N. Roos, J. Schrezenmeir, Lipase inhibitory activity in alcohol extracts of worldwide occurring plants and propolis. Phytotherapy Research: An International Journal devoted to pharmacological and toxicological evaluation of natural product derivatives, 2009. 23(4): p. 585–586
H. Sahin et al., Honey, polen, and propolis extracts show potent inhibitory activity against the zinc metalloenzyme carbonic anhydrase. J. Enzyme Inhib. Med. Chem. 26(3), 440–444 (2011)
Z. Can, Determination of in-vitro Antioxidant, anti-urease, anti-hyaluronidase Activities by Phenolic rich bee Products from Different Region of Turkey (FEB-FRESENIUS ENVIRONMENTAL BULLETIN, 2018), p. 6858
N. Baltas, O. Yildiz, S. Kolayli, Inhibition properties of propolis extracts to some clinically important enzymes. J. Enzyme Inhib. Med. Chem. 31(sup1), 52–55 (2016)
B. Alizadeh-Behbahani et al., Antimicrobial activity of Avicennia marina extracts ethanol, methanol & glycerin against Penicillium digitatum (citrus green mold). Sci. J. Microbiol. 1(7), 147–151 (2012)
L. Drago et al., In vitro antimicrobial activity of propolis dry extract. J. Chemother. 12(5), 390–395 (2000)
S. Stepanović et al., In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol. Res. 158(4), 353–357 (2003)
M. Peixoto et al., Antioxidant and Antimicrobial Activity of Blends of propolis Samples Collected in Different Years (Lwt-Food Science and Technology, 2021), p. 145
A. Alghooneh et al., Application of intelligent modeling to predict the population dynamics of Pseudomonas aeruginosa in Frankfurter sausage containing Satureja bachtiarica extracts. Microb. Pathog. 85, 58–65 (2015)
T.G. Pillai, C.K.K. Nair, K. Janardhanan, Polysaccharides isolated from Ganoderma lucidum occurring in Southern parts of India, protects radiation induced damages both in vitro and in vivo. Environ. Toxicol. Pharmacol. 26(1), 80–85 (2008)
A. Karapetsas et al., Propolis extracts inhibit UV-Induced Photodamage in Human Experimental in Vitro skin models. Antioxidants, 2019. 8(5)
Y. Aliyazicioglu et al., Preventive and protective effects of turkish propolis on H2O2-induced DNA damage in foreskin fibroblast cell lines. Acta Biol. Hung. 62(4), 388–396 (2011)
Acknowledgements
This work was supported by OMU as Research Program and Scientific Research Project (PYO.FEN. 1901.20.001). We thank Yasar Ipek, Dr. Fatih GUL and Dr. M. Nuri ATALAR from Science and Technology Application and Research Center, Igdir University, for their contribution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure statement
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omer, H.H.S., Demirtas, I. & Ozen, T. Investigation of phenolic contents and bioactivities of water-based extracts prepared from cryogenically pulverized Turkish propolis. Food Measure 17, 1586–1601 (2023). https://doi.org/10.1007/s11694-022-01716-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-022-01716-4