Abstract
Flours processed from Indian horse chestnut were subjected to water and ether extraction and studied for physicochemical properties, functional properties and antioxidant potential. Protein, fat, ash and carbohydrate content of native flour was 7.69, 2.85, 2.20 and 80.71 %, respectively. Protein, fat and ash contents decreased significantly (p ≤ 0.05) while the carbohydrate content increased significantly on extraction. Pasting properties of native and extracted flours like peak viscosity, breakdown viscosity and setback viscosity were in the range of 634.3–4125.0, 297.67–2258, and 27.33–1543.4 cP, respectively with significant increase in all the pasting parameters on extraction. Among the functional properties water and oil absorption capacity, emulsion capacity (%) and emulsion stability (%) increased significantly on extraction. Foaming capacity was lost on water extraction but increased significantly on ether extraction. Antioxidant capacity measured as % DPPH inhibition generally decreased on water extraction but increased on ether extraction.
Similar content being viewed by others
Introduction
Indian horse chestnut–IHCN (Aesculus indica Colebr) also known as Himalayian Chestnut belongs to the family Hippocastanaceae. It is locally known as ‘Hanudun’ in Kashmiri and is found in temperate regions of Asia, Europe and America, partially in East Asia, the north western Himalayas and North America [1]. In Asia it is generally found in India, Nepal and Pakistan [2]. It is present in planes as well as hilly areas of Kashmir valley of India. Fruit (seed) set takes place during the month of May and June. Seeds are present in the capsule, each capsule contains single seed. These are about 3.5 cm in diameter with shining black hard rind from outside and lime white cotyledons inside. Seeds ripen in October and these are rich in saponins. Poisonous saponins are poorly absorbed by human body and so pass through without harm. They can be removed by carefully leaching the seeds or flour in running water [1]. Seeds are consumed by wild animals in hilly areas of Kashmir (India).
The seeds, which constitute the edible portion of the fruit, contain 50.5 % moisture. 5.58 % total sugars. The protein and mineral contents are 0.39 and 1.93 %, respectively [3]. Seeds are dried and ground into bitter flour, called tattawakher. The bitterness is caused by saponins, which are rinsed out by thoroughly washing the flour during its preparation. The flour is often mixed with wheat flour to make chapattis (unleavened flat bread) and also to make a halwa (Indian sweet meal) and sometimes is served as a dalia, (a type of porridge or gruel) during fasting periods [4]. Borges et al. [5] reported that chestnuts are characterized by high levels of moisture content and high levels of starch but showed low fat and protein content with significant amount of fiber. The carbohydrate of the chestnuts has been reported to range from 75.3 to 86.3 g/100 g depending on the cultivar.
Functional properties are important in food processing and product formulation. Some of these properties are pasting properties, water/oil binding, emulsification and foam formation. These properties show a great impact on the utilization of ingredients and are very important in the development of functional food. Recent papers report the application of some flours as functional ingredients in some foods such as breads, cakes, biscuits, doughnuts, tortillas, pasta, and snacks, which are now becoming popular in several countries [6, 7].
There is now increasing interest in antioxidant activity (AOA) of phytochemicals present in the diet. IHCN possess AOA which is believed to play a very important role in the body defence system against reactive oxygen species, which are the harmful by-products generated during normal cell aerobic respiration [8]. Increasing intake of dietary antioxidants may help to maintain an adequate antioxidant status and, therefore, the normal physiological function of a living system [9]. In order to use IHCN flour for food uses it is imperative to remove toxic saponins by water leaching and for non food uses its oxidative stability and functional properties could be improved by ether extraction. Therefore, the objectives of the study were to evaluate the effect of water and petroleum ether extraction on composition, functional and antioxidant properties of Indian horse chestnut flour.
Materials and methods
Materials
The Indian horse chestnuts were taken from the lawns of University of Kashmir, J & K, India. Chestnuts were cleaned manually from the dirt, foreign material etc. and stored until further use at 5 °C. All the reagents used in the study were of analytical grade.
Flour preparation
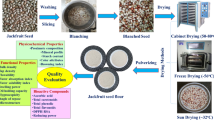
Indian horse chestnut seeds were manually deshelled and the cotyledons were chopped into small pieces of approximately 2 × 2 cm. The pieces were dried at 40 °C to moisture content of about 6 % in a hot air oven (NSW-143; Narang Scientific Works Pvt. Ltd., New Delhi, India) at 40 °C. These were then ground in a laboratory mixer grinder (Sujata, New Delhi, India), sieved through 60-mesh (250 µ) screen to get native flour. For the preparation of water extracted flours, native flour was slurried with distilled water in the ratio of 1:10 and extracted on a magnetic stirrer with continuous agitation by a magnetic bead for 0.5, 1 and 1.5 h. The supernatant was decanted and the sediment was dried in hot air oven (NSW-143; Narang Scientific Works Pvt. Ltd., New Delhi, India) at 40 °C to a moisture content of 6–8 %. Ether extracted flours were prepared by extracting the native flours on soxhlet apparatus for 8 h.
Flours were stored in airtight containers at refrigerated temperature until used for measurement of physicochemical, pasting and functional properties. However, antioxidant properties were measured from alcoholic flour extracts as discussed in antioxidant section.
Physico-chemical properties
Composition
Moisture (925.10), protein (960.10), fat (920.85) and ash (923.03) contents of flours were determined following the methods of AOAC [10].
Bulk density
Bulk density was measured as a ratio of mass to volume according to the method of Wani et al. [11].
Color
The surface colour of flours was measured using Hunter Colour Lab (Hunter Associates Laboratory Inc., Reston, VA, USA). A glass cell containing uniformly sized flour was placed against the light source, covered with a black cover and ‘L’ (Lightness), ‘a’ (red-green) and ‘b’ (yellow-blue) values were recorded [1].
Microscopy
Native and treated flours were analysed on inverted microscope (EAPRIME inverted 900, Leeds, Germany) at ×40. The flour dispersions (1 %) were made in absolute ethyl alcohol and a drop of dispersion was put on glass slide. The slide was made to dry at 45 °C for 1 h in a hot air oven and then viewed under microscope at ×40.
Pasting properties
The pasting properties of the flours were measured using a Rapid Visco Analyzer (RVA-4, Newport Scientific Pty Ltd, Warriewood, Australia). An aqueous dispersion of flour-14 % moisture basis (12.28 %, w/w; 28.5 g total weight) was equilibrated at 50 °C for 1 min, heated at the rate of 12.2 °C/min to 95 °C, held for 2.5 min, cooled to 50 °C at the rate of 11.8 °C/min and again held at 50 °C for 2 min. A constant paddle rotational speed (160 rpm) was used throughout the entire analysis, except for rapid stirring at 960 rpm for the first 10 s to disperse the sample [1].
Functional properties
Water and oil absorption capacity
To determine water and oil absorption capacity (OAC) 1 g (db) of sample was weighed into 25 mL pre-weighed centrifuge tubes and then stirred into 10 mL of double distilled water or refined soyabean oil (Amrit Banaspati Co. Ltd., Rajpura, Punjab, India) for 1 min. The samples were allowed to stand for 30 min and then centrifuged at 2200×g for 30 min. The water or oil released on centrifugation was drained. Water or OAC was expressed as kg of water or oil held per kg of flour sample [1].
Foaming capacity and stability
Aqueous dispersions (2 % w/v db) of the flour were homogenized in a domestic blender (Mittal Electricals, Delhi, India) at 10,000 rpm for 1 min. Foaming capacity (FC) was calculated as the percent increase in volume of the flour dispersion. The foam stability was determined by measuring the foam volume with time and computing half-life [11].
Emulsifying properties
Emulsifying properties were determined by the method adopted by Sridaran et al. [12]. Flour suspension (1 %) was homogenized with 5 mL of refined oil. The emulsions were then centrifuged at 1100×g for 5 min (5810R, Eppendoaf, Hamburg, Germany). Subsequently the height of the emulsified layer and the total contents in the tube were determined. The emulsion capacity was obtained through the following calculation.
Emulsion stability was evaluated by heating the emulsion for 30 min at 80 °C and centrifuging for 5 min at 1100×g.
Antioxidant activity
Total phenolic content
The total phenolic content of the native and treated flour was quantified using the Folin Ciocalteu reagent according to Singleton and Rossi [13]. 0.5 mL of the methanolic extract was adjusted to 3 mL with distilled water and incubated with 0.5 mL of 95 % ethanol and 0.25 mL of Folin’s reagent (1:1 diluted with distilled water) for 5 min at room temperature. Then Na2CO3 (5 %) solution (0.5 mL) was added, mixed and the mixture held for 60 min at room temperature. The absorbance of the solution was then measured at 720 nm against a reagent blank. Gallic acid (0.1 mg/mL) was used as the standard and the phenolic content in the extract was expressed as milligram equivalent of gallic acid (GAE) per 100 g flour.
DPPH scavenging activity
The scavenging activity of DPPH (1,1-dihpenyl-2-picrylhydrazyl) radical was measured according to the modified method of Brand-Williams et al. [14]. Briefly 100μL of each sample extract was added to 1.0 mL of a 0.01 % methanolic solution of DPPH and incubated at 37 °C for 30 min. After incubation, absorbance was measured at 517 nm. The percent inhibition was calculated using the formula.
Reducing power
Reducing power of flour extracts was evaluated according to the method of Oyaizu [15]. A 0.25 mL aliquot of various concentrations of flour extract (1, 10, 50, 100 µg/mL) was mixed with 2.5 mL of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 mL of 1 % potassium ferricyanide. The mixture was then incubated at 50 °C for 20 min. After incubation, 2.5 mL of 10 % trichloro acetic acid (TCA) (W/V) were added and the mixture was centrifuged at 650×g for 10 min. 5 mL aliquot of the upper layer was mixed with 5 mL of distilled water and 1 mL of 0.1 % ferric chloride, and the absorbance of mixture was measured at 700 nm. A higher absorbance indicated a higher reducing power. The percentage reduction is given by the formula
% reduction = Ab of control − Ab of sample × 100/Ab of control
where Ab = absorbance
Statistical analysis
The data reported are averages of triplicate observations. An analysis of variance with a significance level of 5 % was done and Duncan’s test applied to determine differences between means using the commercial statistical package (SPSS, Inc, Chicago, IL, USA)
Results and discussions
Physico-chemical properties
Composition
The chemical composition of IHCN flour is presented in Table 1. The moisture content of native, water extracted (30, 60, 90 min) and ether extracted Indian Horse chestnut flours was 6.55, 6.11–8.27, and 3.21 %, respectively. Protein content of flours varied in the range of 4.15–7.69 %. The highest protein content was observed in native flour and the lowest was observed in water extracted flour (90 min). The decrease in protein content of water extracted flour was proportional to extraction time. Fat content of flours also varied in the range of 0.11–2.85 %. The highest fat content was observed in native flour and the lowest was observed in ether extracted flour. This was due to removal of fat by petroleum ether during treatment. Ash content of flours varied in the range of 0.64–2.54 %. The highest ash content was observed in ether extracted flour and the lowest was observed in water extracted flour (60 and 90 min). Carbohydrate content of flours also varied in the range of 80.71–89.85 %. The highest carbohydrate content was observed in petroleum ether extracted flour and the lowest was observed in case of native flour. Significant (p ≤ 0.05) differences were observed in moisture, protein, fat, ash, and carbohydrate content of native and extracted Indian horse chestnut flours. Protein, fat, ash and carbohydrate of some important chestnut types and cultivars have been reported in the range of 4.88–10.87, 0.49–2.01 and 75.32–86.31 %, respectively [16]. Baraldi et al. [17] reported moisture, protein, lipids and ash contents was 6.97, 2.64, 4.13 and 2.51 % respectively for Aesculus hippocastanum L. seed flour. So, our results fall within the range of reported values.
Color
Hunter ‘L’ value of flours varied from 82.08 to 87.79. The highest ‘L’ value was observed in case of water extracted flour (60 min) and the lowest value was observed in case of petroleum ether extracted flour. ‘a’ and ‘b’ color values of flours varied from 0.43 to 1.51 and 15.69 to 21.48, respectively. The highest ‘b’ value was observed in case of native flour and the lowest value was observed in case of water extracted flour (30 min). Significant (p ≤ 0.05) differences were observed in ‘L’ and ‘b’ color values while as differences were insignificant for ‘a’ values. Higher ‘L’ and lower ‘b’ values of water extracted flours than native and ether extracted flours suggest that they were lighter in colour than native and ether extracted flours. Similar results were observed for sweet chestnuts flours [18].
Bulk density
Bulk density of the IHCN flours is presented in Table 3. Petroleum ether extracted flour has higher (0.70) bulk density than water extracted (0.64) and native (0.65) flour. This might be attributed to removal of lipids which have lower density than carbohydrates and proteins. Wani et al. [11] reported bulk density of black gram flours to range from 0.90 to 1.04 g mL−1.
Microscopy
Micrographs of the native and treated flours showed that the flour consists of starch granules of different sizes and shapes associated with other components like fibers and proteins (Fig. 1). The starch granules were round, elliptical and irregular shapes.
Pasting properties
Pasting properties of IHCN flours are presented in Table 2. Peak viscosity reflects the extent of granule swelling. Peak viscosity (PV) of the flours varied in the range of 634.3–4125.0 cP. The highest value was observed in water extracted flour (90 and 60 min) and the lowest was observed in native flour. The increase in PV of water extracted flour was proportional to extraction time. Significantly (p ≤ 0.05) higher peak viscosity of water extracted flour than rest of the flours could be attributed to its higher starch content and lower protein content. Hamaker and Griffin [19] and Yang and Chang [20] indicated that proteins in rice flour restrict starch granules swelling and reduce the amylogram viscosity. Protein removal was reported to increase the paste viscosity of starch [20]. Native IHCN flours produce only 15 % viscosity of water extracted flours (90 min). This means lesser amount of water extracted flour is required to produce equivalent viscosity. Wani et al. [21] reported PV of 591.0–1030.3 cP for flours from kidney bean cultivars.
Trough viscosity (TV) of the flours varied in the range of 294.0–2003.0 cP. The highest TV was observed in water extracted flour (90 min) and the lowest was observed in ether extracted flour. The TV represents decrease in viscosity following peak viscosity due to disruption of swollen granules by shear and high temperature. TV is influenced by the rate of amylose exudation, granule swelling and amylose–lipid complex formation [22]. Breakdown viscosity (BDV) is a measure of the ease with which the swollen starch granules can be disintegrated. It varied in the range of 297.67–2258 cP. The highest BDV of water extracted flours suggests the degree of stability during cooking is low and it can be made to gel at low temperature and shear. Analysis of variance showed significant (p ≤ 0.05) differences in TV and BDV of IHCN flours.
Final viscosity (FV) of the flours varied in the range of 321.3–3546.4 cP. The highest FV was observed in water extracted flour (90 min) and the lowest was observed in petroleum ether extracted flour. Final viscosity is the ability of starch to form various paste or gel after cooling. Wani et al. [21] reported FV of kidney bean flours in the range of 952.0–1611.7. Kaushal et al. [23] reported FV of rice flour as 4048 cP. Setback viscosity (SBV) of the flours varied in the range of 27.33–1543.4 cP. The highest SBV was observed in case of water extracted flour (90 min) and the lowest was observed in ether extracted flour. SBV of taro and rice flours was reported as 487 and 1870 cP, respectively [23]. IHCN flours are thus better than rice and taro flours as they have lower setback. Significant (p ≤ 0.05) differences were observed in peak and SBV of IHCN flours. The setback and final viscosity are largely due to re-ordering or polymerisation of leached amylose & long linear amylopectin [24, 25].
Pasting temperature (PT) of the flours varied significantly (p ≤ 0.05) in the range of 63.83–66.76 °C. The highest was observed in native flour and the lowest was observed in water extracted flour (90 min). This indicates that native flour cooks at a higher temperature and therefore likely to scorch during cooking [26]. This means frequent stirring would be necessary during cooking. Lower PT means faster swelling.
Functional properties
Water and oil absorption capacity
Water and OAC of IHCN flours are presented in Table 3. Water absorption capacity (WAC) of the flours varied in the range of 2.40–3.23 g/g. The highest WAC was observed in water extracted flour (60 min) and the lowest was observed in petroleum ether extracted flour. Water extracted flours had significantly (p ≤ 0.05) higher WAC than native and ether extracted flours. This indicates that water extracted flours has good ability to bind water. Wani et al. [11] reported WAC of black gram flours from 2.9 to 3.1 g/g. The major chemical components that enhance the WAC of flours are proteins and carbohydrates, because these constituents contain hydrophilic parts, such as polar or charged side chains [27]. Despite the higher carbohydrate content of ether extracted flour, water extracted flour had higher WAC. This may be due to the reason that water soluble proteins are present in ether extracted flour which act as competitors for other proteins and carbohydrates for water absorption, resulting in less water absorption as they are removed during decanting after centrifugation. Erdogdu et al. [28] reported that water soluble fractions of wheat including albumins, globulins, and pentosans compete with starch to absorb water. On centrifugation, albumins, globulins, and other water soluble proteins are transferred to the supernatant and are removed during the process of decantation [29]. Hoover and Sosulski [30] reported that WAC is influenced by the degree of inter molecular association between the starch polymers because of associative forces such as hydrogen and covalent bonding.
OAC of the flours varied in the range of 3.13–3.64 g/g. Significant (p ≤ 0.05) differences were not observed in OAC of flours. OAC of black gram flours has been reported from 2.1 to 2.2 g/g (Wani et al. [21]. OAC is another important functional property of flours, because it plays an important role in enhancing the mouth feel and retaining the flavour [31]. The major chemical component affecting OAC is protein, which is composed of both hydrophilic and hydrophobic parts. Non-polar amino acid side chains can form hydrophobic interactions with hydrocarbon chains of lipid.
Foaming capacity and stability
FC and stability of IHCN flours are presented in Table 3. The FC of the flours varied in the range of 0–60.83 %. The highest FC was observed in case of petroleum ether extracted flour and the lowest was observed in water extracted flours. This may be attributed to loss of proteins especially albumins in water extracted flours. Proteins form a continuous cohesive film around the air bubbles in the foam. Proteins in the dispersion result in the lowering of the surface tension at the water–air interface, thus creating FC. FC of black gram flours at pH 8 has been reported from 112.0 to 119.6 % [11].
Foam stability is important since the usefulness of whipping agents depends on their ability to maintain the whip as long as possible. Foam stability (FS) of IHCN flours measured as half life of foam varied from 19.17 to 23.0 min (Table 3). Significantly higher FS was observed in ether extracted flour than native flour. This may be attributed to the presence of higher soluble proteins in ether extracted flour (Tables 4, 5).
Antioxidant activity
Total phenolic content
The total phenolic content of the different extracts was expressed in terms of GAE per gram flour from which the extract was obtained (Table 1). Among the extracts prepared with different time of extraction, the total phenolic content increases with time and the horse chestnut flour extracted with ether was having highest phenolic content.
DPPH scavenging activity
Estimation of the AOA by scavenging of stable radicals such as the chromogen radical DPPH in inorganic media has been extensively used for comparison of homogeneous series of antioxidants. This procedure measures the hydrogen donating capacity of the target substances in a methanolic media. Antioxidant molecules chelate the free radical by hydrogen donation, the color from the DPPH assay solution (purple) becomes light yellow resulting in a decrease in absorbance at 517 nm [32]. The DPPH scavenging activity increased with increase in extraction time and was highest in the flour samples extracted with ether. The DPPH radical scavenging activity was shown dose dependent behaviour. Increasing the concentration of extract led to significant increase in DPPH radical scavenging activity. The extract from native flour had 23.5 % DPPH free radical scavenging at 10 mg/mL and it increased up to 81.66 % when 40 mg/mL concentration was used. Similarly, water extracted flour (30, 60 and 90 min) had DPPH free radical scavenging activity of 19.97, 27.10 and 23.03 %, respectively while increasing the concentration up to 40 mg/mL significantly increased the radical scavenging activity by 63.48, 77.61 and 84.60 %, respectively. Also, increasing the concentration of extract from flour obtained after ether extraction was exhibited a sharp increased in radical scavenging activity 39.31 % (10 mg/mL) to 95.80 (40 mg/mL). The DPPH radical scavenging activity was varied significantly among flour obtained after extraction with different solvents. The highest and the lowest radical scavenging activity was observed for flour obtained after ether extract and obtained after 30 min extraction with water, respectively for all the concentration. The increased AOA after ether extraction may be due to the removal of fat and thus the antioxidant compounds were extracted easily with methanol and exhibited higher free radical scavenging activity. Interestingly, increasing the extraction time from 30 to 60 min, (with water) increased the radical scavenging activity and further increasing the extraction time to 90 min was exhibited a significant decrease in radical scavenging activity. The results are also in agreement with those reported by Sato et al. [33] for Japanese horse chestnut. Stajner et al. [34] was reported the DPPH free radical scavenging activity of horse chestnut seeds 36.5 %. Pereira et al. [35] reported as high as 92.6 % radical scavenging capacity for walnut.
Reducing power
The reducing power is also an indicator of AOA. The electron donor compounds are considered as a reducing agent and can reduce the oxidised intermediates of the lipid peroxidation reactions therefore there may be primary or secondary antioxidants. The reducing power of an antioxidant compound is associated with the presence of reductones. Further, the antioxidant capacity of reductones is based on the breaking of the free radical chain reaction by donating a hydrogen atom, and to prevent peroxide formation [30, 36]. Similar to DPPH radical scavenging activity, the reducing power of extracts from different types of flour were exhibited concentration dependent activity. The reducing power of flour from native horse chestnut showed the reducing power 25.45 % (10 mg/ml) while flour obtained after water extraction for 30 min exhibited a significant decrease (19.97 %). Increasing the extraction time with water, from 30 to 60 min led to a significant increase in reducing power up to 27.10 % but further increasing the extraction time to 90 min exhibited a significant decrease in reducing power. On the other hand, the flour obtained after extraction with ether showed the highest reducing power (39.31 %) at all the extract concentration levels. Oliveira et al. [37] reported the reducing power (EC50) ranging from 0.50 to 0.70 of walnut green hull. The extract from different cultivars of walnut was showed the reducing power (EC50) ranging from 0.19 to 0.26.
Conclusion
The present study showed significant differences in physicochemical properties between native and extracted flours. Water extracted flours had high viscosity than native flours suggesting their use as thickening agents at low concentration. Low SBV of flours implies, they can be used in formulations where starch stability is required at low temperature. Water extracted flours do not possess foaming properties which means saponins have been removed from the flour. Besides they displayed higher emulsion capacity than native flours. Native flours also displayed good antioxidant properties, which however reduced on water extraction and increased on ether extraction.
References
I.A. Wani, M. Jabeen, H. Geelani, F.A. Masoodi, I. Saba, S. Muzaffar, Effect of gamma irradiation on physicochemical properties of Indian horse chestnut (Aesculus indica) starch. Food Hydrocoll. 35, 253–263 (2014)
Singh B. Simple process for obtaining beta Aescin from Indian horse chestnut. United States patent application pub. no. US 2006/0030697 (2006)
C. Parmar, M.K. Kaushal, Aesculus indica, Wild Fruits (Kalyani Publishers, New Delhi, 1982), p. 6e9
G. Singh, P. Kachroo (1976), Forest flora of Srinagar. cf http://www.ibiblio.org/pfaf/cgi-bin/arr_html.Aesculus indica
O. Borges, B. Goncalves, J.S. Carvalho, P. Correia, A.P. Silva, Nutritional quality of chestnuts (Castanea sativa Mill.) cultivars from Portugal. Food Chem. 106, 976–984 (2008)
A.A. Anton, K.A. Ross, O.M. Lukow, R.G. Fulcher, S.A. Arntfield, Influence of added bean flour (Phaseolus vulgaris L.) on some physical and nutritional properties of wheat flour tortillas. Food Chem. 109, 33–41 (2008)
J. Han, J.A.M. Janz, M. Gerlat, Development of gluten-free cracker snacks using pulse flours and fractions. Food Res. Int. 43(2), 627–633 (2010)
J.M.C. Gutteridge, B. Halliwell, Free Radicals in Biology and Medicine (Oxford University Press, Oxford, 2000)
C. Kaur, H. Kapoor, Review: antioxidants in fruits and vegetables—the millennium’s health. Int. J. Food Sci. Technol. 36, 703–725 (2001)
AOAC, Official Methods of Analysis (Association of official Analytical chemistry, Arlington, 1990)
I.A. Wani, D.S. Sogi, B.S. Gill, Physicochemical and functional properties of flours from three Black gram (Phaseolus mungo L.) cultivars. Int. J. Food Sci. Technol. 48, 771–777 (2013)
A. Sridaran, A.A. Karim, R. Bhat, Pithecellobium jiringa legume flour for potential food applications: studies on their physico-chemical and functional properties. Food Chem. 130, 528–535 (2012)
V.L. Singleton, J.A. Rossi, Colorimetry of total phenols with phospho molybdiphosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158 (1965)
W. Brand-Williams, M.E. Cuvelier, C. Berset, Use of free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 28, 25–30 (1995)
M. Oyaizu, Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 44, 307–315 (1986)
U. Erturk, C. Mert, A. Soylu, Chemical composition of fruits of some important chestnut cultivars. Braz. Arch. Biol. Technol. 49, 183–188 (2006)
C. Baraldi, L.M. Bodecchi, M. Cocchi, C. Durante, G. Ferrari, G. Foca, M. Grandi, A. Marchetti, M. Tassi, A. Ulrici, Chemical composition and characterisation of seeds from two varieties (pure and hybrid) of Aesculus hippocastanum. Food Chem. 104(1), 229–236 (2007)
P. Correia, L. Cruz-Lopes, L. Beirao-da-Costa, Morphology & structure of chestnut starch isolated by alkali & enzymatic methods. Food Hydrocoll 28, 313–319 (2012)
B. Hamaker, V.K. Griffin, Effect of disulfide bond-containing protein on rice starch gelatinization and pasting. Cereal Chem. 70, 377–380 (1993)
C.H. Yang, W.H. Chang, Effects of protein and lipid binding to starch on the physicochemical and pasting properties of rice flour. J. Food Sci. Agric. Chem. 1, 277–285 (1999)
I.A. Wani, D.S. Sogi, A.A. Wani, B.S. Gill, Physico-chemical and functional properties of flours from Indian kidney bean (Phaseolus vulgaris L.) cultivar. LWT Food Sci. Technol. 53, 278–284 (2013)
A.A. Wani, P. Singh, M.A. Shah, U. Schweiggert-Weisz, K. Gul, I.A. Wani, Rice starch diversity: effect on structural, morphological, thermal, and physicochemical properties—a review. Compr. Rev. Food Sci. Food Saf. 11(5), 417–436 (2012)
P. Kaushal, V. Kumar, H.K. Sharma, Comparative study of physicochemical, functional, antinutritional and pasting properties of taro (Colocasia esculenta L.), rice (Oryza sativa L.) flour, pigeonpea (Cajanus cajan L.) flour and their blends. LWT-Food. Sci. Technol. 48(1), 59–68 (2012)
J.O. Abu, K.G. Duodu, A. Minnaar, Effect of g-irradiation on some physicochemical and thermal properties of cowpea (Vigna unguiculata L.) starch. Food Chem. 95, 386–393 (2006)
D. Wu, Q. Shu, Z. Wang, Y. Xia, Effect of gamma irradiation on starch viscosity and physicochemical properties of different rice. Radiat. Phys. Chem. 65(1), 79–86 (2002)
H.F. Zobel, Molecules to granules: a comprehensive starch review. Starch 40, 44–50 (1988)
J.C. Hodge, E.M. Osman, Carbohydrates, principles of food science, Part I, in Food chemistry, ed. by R.O. Fennema (Marcel Dekker Inc, New York, 1976), pp. 97–200
N. Erdogdu, Z. Czuchajowska, Y. Pomeranz, Wheat flour and defatted milk fractions characterized by differential scanning calorimetry. I. DSC of flour and milk fractions. Cereal Chem. 72(l), 70–75 (1995)
R.C. Hoseney, K.F. Finney, M.D. Shogren, Y. Pomeranz, Functional (Breadmaking) and biochemical properties of wheat flour components. Role of water-solubles. Wheat Flour Compon. Prop. II 46, 117–125 (1969)
R. Hoover, F. Sosulski, Effect of cross-linking on functional properties of legume starches. Starch/Starke 38, 149–155 (1986)
J.E. Kinsella, Functional properties of proteins in foods: a survey. Crit. Rev. Food Sci. Nutr. 7, 219–232 (1976)
P. Sharma, H.S. Gujral, B. Singh, Antioxidant activity of barley as affected by extrusion cooking. Food Chem. 131(4), 1406–1413 (2012)
I. Sato, T. Suzuki, H. Kobasashi, S. Tsuda, Antioxidative and antigenotoxic effect of Japanase horse chestnut (Aesculus turbinate) seeds. J. Vet. Med. Sci. 67, 731–734 (2005)
D. Stajner, B.M. Popovic, D. Calic, M. Stajner, Comparative Study of Antioxidant Status in Androgenic Embryos of Aesculus hippocastanum and Aesculus flava. Sci. World J. (2014). doi:10.1155/2014/767392
J.A. Pereira, I. Oliveira, A. Sousa, I.C.F.R. Ferreira, A. Bento, L. Estevinho, Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 46, 2103–2111 (2008)
P. Sharma, H.S. Gujral, Effect of sand roasting and microwave cooking on antioxidant activity of barley. Food Res. Int. 44(1), 235–240 (2011)
I. Oliveira, A. Sousa, I.C.F.R. Ferreira, A. Bento, L. Estevinho, J.A. Pereira, Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 46, 2326–2331 (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shafi, S., Wani, I.A., Gani, A. et al. Effect of water and ether extraction on functional and antioxidant properties of Indian horse chestnut (Aesculus indica Colebr) flour. Food Measure 10, 387–395 (2016). https://doi.org/10.1007/s11694-016-9317-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-016-9317-0