Abstract
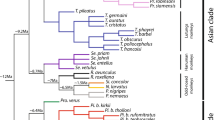
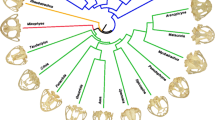
Two bat families, the leaf-nosed (Phyllostomidae) and fruit bats (Pteropodidae), have independently evolved the ability to consume plant resources. However, despite their similar ages, species richness and the strong selective pressures placed on the evolution of skull shape by plant-based foods, phyllostomids display more craniofacial diversity than pteropodids. In this study, we used morphometrics to investigate the distribution of palate variation and the evolution of palate diversity in these groups. We focused on the palate because evolutionary alterations in palate morphology are thought to underlie much feeding specialization in bats. We hypothesize that the distribution of palate variation differs in phyllostomids and pteropodids, and that the rate of palate evolution is higher in phyllostomids than pteropodids. The results suggest that the overall level of palate integration is higher in adult populations of pteropodids than phyllostomids but that the distribution of palate variation is otherwise generally conserved among phyllostomids and pteropodids. Furthermore, the results are consistent with these differences in palate integration likely having a developmental basis. The results also suggest that palate evolution has occurred significantly more rapidly in phyllostomids than pteropodids. These findings are consistent with a scenario in which the greater integration of the pteropodid palate has limited its evolvability.




Similar content being viewed by others
References
Adams, D. C. (2014). Quantifying and comparing phylogenetic evolutionary rates for shape and other high-dimensional phenotypic data. Systematic Biology, 63, 166–177.
Adams, D. C., & Otárola-Castillo, E. (2013). Geomorph: An r package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution, 4, 393–399.
Almeida, F. C., Giannini, N. P., DeSalle, R., & Simmons, N. B. (2011). Evolutionary relationships of the old world fruit bats (chiroptera, pteropodidae): Another star phylogeny? BMC Evolutionary Biology, 11, 281.
Bell, E., Andres, B., & Goswami, A. (2011). Integration and dissociation of limb elements in flying vertebrates: a comparison of pterosaurs, birds and bats. Journal of Evolutionary Biology, 24, 2586–2599.
Bennett, V., & Goswami, A. (2011). Does developmental strategy drive limb integration in marsupials and monotremes? Mammalian Biology, 76, 79–83.
Bennett, C. V., & Goswami, A. (2013). Statistical support for the hypothesis of developmental constraint in marsupial skull evolution. BMC Biology, 11, 52.
Cheverud, J. M., Wagner, G. P., & Dow, M. M. (1989). Methods for the comparative analysis of variation patterns. Systematic Zoology, 38, 201–213.
Dryden, I. L., & Mardia, K. V. (1998). Statistical shape analysis. Chichester: Wiley.
Dumont, E. R. (1997). Cranial shape in fruit, nectar and exudate feeders. American Journal of Physical Anthropology, 102, 187–202.
Dumont, E. R. (1999). The effect of food hardness on feeding behaviour in frugivorous bats (phyllostomidae): An experimental study. Journal of Zoology, 248, 219–229.
Dumont, E. R. (2004). Patterns of diversity in cranial shape among plant-visiting bats. Acta Chiropterologica, 6, 59–74.
Dumont, E. R. (2006). The correlated evolution of cranial morphology and feeding behavior in New World fruit bats. In A. Zubaid, G. F. McCracken, & T. H. Kunz (Eds.), Functional and evolutionary ecology of bats (pp. 160–177). Chicago: University of Chicago Press.
Dumont, E. R. (2007). Feeding mechanisms in bats: Variation within the constraints of flight. Integrative and Comparative Biology, 47, 137–146.
Dumont, E. R., Davalos, L. M., Goldberg, A., Santana, S. E., Rex, K., & Voigt, C. C. (2012). Morphological innovation, diversification and invasion of a new adaptive zone. Proceedings of the Royal Society B Biological Sciences, 279, 1797–1805.
Dumont, E. R., & O’Neil, R. (2004). Fruit hardness, feeding behavior, and resource partitioning in Old World fruit bats (family pteropodidae). Journal of Mammalogy, 85, 8–14.
Escoufier, Y. (1973). Le traitement des variables vectorielles. Biometrics, 29, 751–760.
Ferrarezzi, H., & Gimenez, E. D. A. (1996). Systematic patterns and the evolution of feeding habits in chiroptera (archonta: Mammalia). Journal of Computational Biology, 1, 75–94.
Freeman, P. W., (1998) Form, function, and evolution in skulls and teeth of bats. In: T. H. Kunz, P. A. Racey (Eds). Bat Biology and Conservation. Smithsonian Institution Press (pp. 140–156).
Gardner, A. L. (1977). Feeding habits. In R. J. Baker, J. K. Jones, & D. C. Carter (Eds.), Biology of Bats of the New World family phyllostomidae, part 2 (pp. 293–350). Lubbock: Texas Tech Press.
Gerber, S., Eble, G. J., & Neige, P. (2008). Allometric space and allometric disparity: A developmental perspective in the macroevolutionary analysis of morphological disparity. Evolution, 62, 1450–1457.
Gómez-Robles, A., & Polly, P. D. (2012). Morphological integration in the hominin dentition: Evolutionary, developmental, and functional factors. Evolution, 66, 1024–1043.
Goswami, A. (2006). Cranial modularity shifts during mammalian evolution. American Naturalist, 168, 270–280.
Goswami, A., & Polly, P. D. (2010). The influence of modularity on cranial morphological disparity in carnivora and primates (mammalia). PLoS One, 5, e9517.
Goswami, A., Smaers, J., Soligo, C., & Polly, P. D. (2014). The macroevolutionary consequences of phenotypic integration: from development to deep time. Philosophical Transaction of the Royal Society B, 369, 20130254.
Hallgrímsson, B., Willmore, K., Dorval, C., & Cooper, D. M. (2004). Craniofacial variability and modularity in macaques and mice. Journal of Experimental Zoology Part B Molecular and Developmental Evolution, 302, 207–225.
Hansen, T. F., & Houle, D. (2008). Measuring and comparing evolvability and constraint in multivariate characters. Journal of Evolutionary Biology, 21, 1201–1219.
Hunt, G. (2007). Evolutionary divergence in directions of high phenotypic variance in the ostracode genus Poseidonamicus. Evolution, 61, 1560–1576.
Irschick, D. J., Albertson, R. C., Brennan, P., Podos, J., Johnson, N. A., Patek, S., et al. (2013). Evo-devo beyond morphology: From genes to resource use. Trends in Ecology & Evolution, 28, 267–273.
Jones, K. E., Bininda-Emonds, O. R., & Gittleman, J. L. (2005). Bats, clocks, and rocks: diversification patterns in Chiroptera. Evolution, 59, 2243–2255.
Jones, K. E., Purvis, A., MacLarnon, A., Binida-Emonds, O. R. P., & Simmons, N. B. (2002). A phylogenetic supertree of the bats (mammalia: chiroptera). Biological Reviews of the Cambridge Philosophical Society, 77, 223–259.
Kavanagh, K. D., Evans, A. R., & Jernvall, J. (2007). Predicting evolutionary patterns of mammalian teeth from development. Nature, 449, 427–432.
Kelly, M., & Sears, K. E. (2011). Reduced integration in marsupial limbs and the implications for mammalian evolution. Biological Journal of the Linnaean Society, 102, 22–36.
Klingenberg, C. P. (2003a). Developmental constraints, modules, and evolvability. In B. Hallgrímsson & B. K. Hall (Eds.), Variation: A central concept in biology (pp. 219–249). Oxford: Elsevier.
Klingenberg, C. P. (2003b). Developmental instability as a research tool: Using patterns of fluctuating asymmetry to infer the developmental origins of morphological integration. In M. Polak (Ed.), Developmental instability: Causes and consequences (pp. 427–442). New York: Oxford University Pres.
Klingenberg, C. P. (2003c). A developmental perspective on developmental instability: Theory, models and mechanisms. In M. Polak (Ed.), Developmental instability: Causes and consequences (pp. 14–34). New York: Oxford University Press.
Klingenberg, C. P. (2005). Developmental constraints, modules and evolvability. In B. Hallgrímsson & B. K. Hall (Eds.), Variation (pp. 219–247). San Diego: Academic Press.
Klingenberg, C. P. (2008). Morphological integration and developmental modularity. Annual review of ecology, evolution, and systematics, 39, 115–132.
Klingenberg, C. P. (2009). Morphometric integration and modularity in configurations of landmarks: Tools for evaluating a priori hypotheses. Evolution & Development, 11, 405–421.
Klingenberg, C. P. (2011). MorphoJ: An integrated software package for geometric morphometrics. Molecular Ecology Resources, 11, 353–357.
Klingenberg, C. P., Barluenga, M., & Meyer, A. (2002). Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution, 56, 1909–1920.
Klingenberg, C. P., & Gidaszewski, N. A. (2010). Testing and quantifying phylogenetic signals and homoplasy in morphometric data. Systematic Biology, 59, 245–261.
Klingenberg, C. P., & McIntyre, G. S. (1998). Geometric morphometrics of developmental instability: Analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution, 52, 1363–1375.
Marcus, L., Hingst-Zaher, E., & Zaher, H. (2000). Application of landmark morphometrics to skulls representing the orders of living mammals. The Italian Journal of Mammalogy, 1, 6–25.
Marriog, G., & Cheverud, J. M. (2001). A comparison of phenotypic variation and covariation patterns and the role of phylogeny, ecology, and ontogeny during cranial evolution of New World monkeys. Evolution, 55, 2576–2600.
Marroig, G., Shirai, L., Porto, A., de Oliveira, F. B., & De Conto, V. (2009). The evolution of modularity in the mammalian skull II: Evolutionary consequences. Evolutionary Biology, 36, 136–148.
McAdams, H. H., & Arkin, A. (1999). It’s a noisy business! genetic regulation at the nanomolar scale. Trends in Genetics, 15, 65–69.
Moller, A. P., & Swaddle, J. P. (1997). Asymmetry, developmental stability, and evolution. Oxford: Oxford University Press.
Myers, P., Espinoza, R., Parr, S., Jones,T., Hammond,G. S., Dewey, T. A., (2013) The Animal Diversity Web (online). http://animaldiversity.org.
Palmer, A. R., & Strobeck, C. (1986). Fluctuating asymmetry: Measurement, analysis, patterns. Annual Review of Ecology and Systematics, 17, 391–421.
Parsons, K. J., Marquez, E., & Albertson, R. C. (2012). Constraint and opportunity: The genetic basis and evolution of modularity in the cichlid mandible. The American Naturalist, 179, 64–78.
Pavlicev, M., Cheverud, J., & Wagner, G. (2009). Measuring morphological integration using eigenvalue variance. Evolutionary Biology, 36, 157–170.
Polly, P. D. (2005). Development and phenotypic correlations: The evolution of tooth shape in Sorex araneus. Evolution & Development, 7, 29–41.
Porto, A., de Oliveira, F. B., Shirai, L., De Conto, V., & Marroig, G. (2009). The evolution of modularity in the mammalian skull 1: Morphological integration patterns and magnitudes. Evolutionary Biology, 36, 118–135.
Raff, R. A. (1996). The shape of life: genes, development, and the evolution of animal form. Chicago: University of Chicago Press.
Salazar-Ciudad, I., & Jernvall, J. (2010). A computational model of teeth and the developmental origins of morphological variation. Nature, 464, 583–586.
Santana, S. E., Dumont, E. R., & Davis, J. L. (2010). Mechanics of bite force production and its relationship to diet in bats. Functional Ecology, 24, 776–784.
Santana, S. E., Grosse, I. R., & Dumont, E. R. (2012). Dietary hardness, loading behavior, and the evolution of skull form in bats. Evolution, 66, 2587–2598.
Sears, K. E. (2004). Constraints on the morphological evolution of marsupial shoulder girdles. Evolution, 58, 2353–2370.
Sears, K. E. (2014). Differences in growth generate the diverse palate shapes of New World leaf-nosed bats (order chiroptera, family phyllostomidae). Evolutionary Biology, 41, 12–21.
Sears, K. E., Goswami, A., Flynn, J. J., & Niswander, L. (2007). The correlated evolution of Runx2 tandem repeats, transcriptional activity and facial length in carnivora. Evolution & Development, 9, 555–565.
Simmons, N. B. (2005). Order chiroptera. In D. E. Wilson & D. M. Reeder (Eds.), Mammal species of the world: A taxonomic and geographic reference (pp. 313–529). Baltimore: Johns Hopkins University Press.
Sztencel-Jabłonka, A., Jones, G., & Bogdanowicz, W. (2009). Skull morphology of two cryptic bat species: Pipistrellus pipistrellus and P. pygmaeu—A 3D geometric morphometrics approach with landmark reconstruction. Acta Chiropterologica, 11, 113–126.
Teeling, E. C., Springer, M. S., Madsen, O., Bates, P., O’Brien, S. J., & Murphy, W. J. (2005). A molecular phylogeny for bats illuminates biogeography and the fossil record. Science, 307, 580–584.
Wagner, G. (1984). On the eigenvalue distribution of genetic and phenotypic dispersion matrices: Evidence for a non-random origin of quantitative genetic variation. Journal of Mathematical Biology, 21, 77–95.
Wagner, G. P. (1988). The influence of variation and developmental constraints on the rate of multivariate phenotypic evolution. Journal of Evolutionary Biology, 1, 45–66.
Wagner, G. (1990). A comparative study of morphological integration in Apis mellifera (insecta, hymenoptera). Journal of Zoological Systematics and Evolutionary Research, 28, 48–61.
Wagner, G. P. (1996). Homologues, natural kinds and the evolution of modularity. American Zoologist, 36, 36–43.
Willmore, K. E., Klingenberg, C. P., & Hallgrímsson, B. (2005). The relationship between fluctuating asymmetry and environmental variance in rhesus macaque skulls. Evolution, 59, 898–909.
Young, N. M. (2006). Function, ontogeny and canalization of shape variance in the primate scapula. Journal of Anatomy, 209, 623–636.
Young, N. M., Wagner, G. P., & Hallgrímsson, B. (2010). Development and the evolvability of human limbs. Proceedings of the National Academy of Sciences of the United States of America, 107, 3400–3405.
Zelditch, M. L., Mezey, J., Sheets, H. D., Lundrigan, B. L., & Garland, T. (2006). Developmental regulation of skull morphology II: Ontogenetic dynamics of covariance. Evolution & Development, 8, 46–60.
Zelditch, M. L., Swiderski, D. L., Sheets, H. D., & Fink, W. L. (2004). Geometric morphometrics for biologists: A primer. Boston: Elsevier Academic Press.
Acknowledgments
We thank Bill Stanley and the staff at the Field Museum of Natural History for their generous specimen loans. We also thank the Wildlife Section, Forestry Division, Ministry of Agriculture, Land, and Marine Resources (currently in the Ministry of Public Utilities and the Environment) of the Republic of Trinidad and Tobago for the issuance of required collecting and export permits. We thank J. Marcot and B. Dumont for discussions that improved the manuscript, and L. Powers for preparation of bat skeletons. We thank A. Suarez for loan of the Canon used in this study. Finally, we thank Richard Behringer, Merla Hübler, Dan Urban and Simeon Williams for field assistance and collection of tissues in Trinidad. This work was supported by NIH NRSA F32 HD050042-01 and NSF IOS-1257873 to KS and NSF IOS-1255926 to CC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sorensen, D.W., Butkus, C., Cooper, L.N. et al. Palate Variation and Evolution in New World Leaf-Nosed and Old World Fruit Bats (Order Chiroptera). Evol Biol 41, 595–605 (2014). https://doi.org/10.1007/s11692-014-9291-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-014-9291-6