Abstract
Purpose
The tick-borne protozoa piroplasms, including Theileria and Babesia, are the cause of substantial economic losses to the livestock industry. However, in southern Qinghai province, China, there are limited information on the molecular characteristics of piroplasms. This study therefore aimed at determining the prevalence and genetic diversity of piroplasms.
Methods
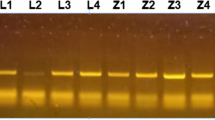
In order to detect piroplasms, we examined 330 yaks and 236 Tibetan sheep blood samples by nested PCR. The differences in piroplasms prevalence in relation to different risk factors was analyzed using SPSS 26. Phylogenetic analysis based on 18S rRNA sequences was inferred using MEGA 7.
Results
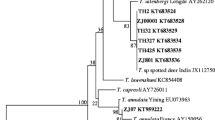
During this study, Theileria spp. were detected in 33.6% (111/330) of yaks and 94.1% (222/236) of Tibetan sheep, but no Babesia was identified. Importantly, a comparison study revealed that T. ovis infection was highly prevalent in sheep (94.1%) but infrequent in yaks (6.1%), while T. sinensis was host-specific to yaks with an infection rate of 27.6%. In addition, male animals were more likely to be infected by T. sinensis and female animals were more likely to be infected by T. ovis. And animals below 4000 m areas reported a higher infection rate with T. sinensis (26.1% vs. 2.9%, p < 0.001). Alongside these differences in prevalence, we found a significantly higher T. sinensis infection rate in separated-grazing livestock (22.2% vs. 3.7%, p < 0.001), while mixed-grazing ruminants had a higher T. ovis infection rate (50.0% vs. 39.0%, p = 0.014). Furthermore, sequence analysis revealed that the 18S rRNA sequences obtained in this study shared 86.9–100.0% identities with each other and they were clustered into T. sinensis or T. ovis.
Conclusion
To our knowledge, this is the first report of T. sinensis in Qinghai region. In addition, high prevalence of the generally sub-clinical T. ovis in sheep indicates extensive exposure to ticks and transmission of tick-borne pathogens with a significant economic impact. This study provides insights into the distribution and genetic diversity of Theileria in China.


Similar content being viewed by others
References
Taylor MA, Coop RL, Wall RL (2015) Part 1: General parasitology including taxonomy, diagnosis, antiparasitics. In: Taylor MA, Coop RL, Wall RL (eds) Veterinary parasitology. Wiley, pp 313–335
Keroack CD, Elsworth B, Duraisingh MT (2019) To kill a piroplasm: genetic technologies to advance drug discovery and target identification in Babesia. Int J Parasitol 49(2):153–163. https://doi.org/10.1016/j.ijpara.2018.09.005
Cruthers LR (2019) Chapter 5-protozoa. In: Marchiondo AA, Cruthers LR, Fourie JJ (eds) Parasiticide screening, vol 1. Academic Press, pp 379–540
Qin G, Li Y, Liu J, Liu Z, Yang J, Zhang L, Liu G, Guan G, Luo J, Yin H (2016) Molecular detection and characterization of Theileria infection in cattle and yaks from Tibet Plateau Region China. Parasitol Res 115(7):2647–2652. https://doi.org/10.1007/s00436-016-5011-8
Qi M, Cui Y, Song X, Zhao A, Bo J, Zheng M, Ning C, Tao D (2018) Common occurrence of Theileria annulata and the first report of T. ovis in dairy cattle from southern Xinjiang China. Ticks Tick Borne Dis 9(6):1446–1450. https://doi.org/10.1016/j.ttbdis.2018.06.017
Wang J, Liu J, Yang J, Wang X, Li Z, Jianlin X, Li X, Xiang Q, Li Y, Liu Z, Luo J, Guan G, Yin H (2019) The first molecular detection and genetic diversity of Babesia caballi and Theileria equi in horses of Gansu province China. Ticks Tick Borne Dis 10(3):528–532. https://doi.org/10.1016/j.ttbdis.2019.01.003
Li Y, Guan G, Ma M, Liu J, Ren Q, Luo J, Yin H (2011) Theileria ovis discovered in China. Exp Parasitol 127(1):304–307. https://doi.org/10.1016/j.exppara.2010.07.002
Taylor MA, BSc RLC, Wall RL (2015) Parasites of horses. In: Taylor MA, Coop RL, Wall RL (eds) Veterinary parasitology. Wiley, USA, pp 524–564
Chaisi ME, Collins NE, Oosthuizen MC (2014) Phylogeny of Theileria buffeli genotypes identified in the South African buffalo (Syncerus caffer) population. Vet Parasitol 204(3–4):87–95. https://doi.org/10.1016/j.vetpar.2014.06.001
Jia L, Zhao S, Xie S, Li H, Wang H, Zhang S (2020) Molecular prevalence of Theileria infections in cattle in Yanbian, north-eastern China. Parasite 27:19
Bai Q, Liu G, Han G (1995) An unidentified Theileria sp. infective to cattle in China. Chin J Vet Sci 15:16–21
Qi B (2002) Theileria Sinensis sp. nov: a new species of bovine theileria—classical taxonomic studies. Chin J Anm Vet Sci 33(2):185–190
Liu J, Guan G, Liu Z, Liu A, Ma M, Bai Q, Yin H, Luo J (2013) Additional data for a new Theileria sp. from China based on the sequences of ribosomal RNA internal transcribed spacers. Exp Parasitol 133(2):217–221. https://doi.org/10.1016/j.exppara.2012.11.023
Taylor MA, Coop RL, Wall RL (2015) Parasites of sheep and goats. In: Taylor MA, Coop RL, Wall RL (eds) Veterinary parasitology. Wiley, pp 436–523
Li J, Jian Y, Jia L, Galon EM, Benedicto B, Wang G, Cai Q, Liu M, Li Y, Ji S, Tumwebaze MA, Ma L, Xuan X (2020) Molecular characterization of tick-borne bacteria and protozoans in yaks (Bos grunniens), Tibetan sheep (Ovis aries) and Bactrian camels (Camelus bactrianus) in the Qinghai-Tibetan Plateau area China. Ticks Tick Borne Dis 11(5):101466. https://doi.org/10.1016/j.ttbdis.2020.101466
Abdullah HH, Aboelsoued D, Farag TK, Megeed KNA, Abdel-Shafy S, Parola P, Raoult D, Mediannikov O (2020) Molecular characterization of some equine vector-borne pathogens and identification of their vectors in Egypt. https://doi.org/10.21203/rs.3.rs-26089/v1
Neitz WO (1972) The experimental transmission of Theileria ovis by Rhipicephalus evertsi mimeticus and R. bursa. Onderstepoort J Vet Res 39(2):83–86
Li K, Shahzad M, Zhang H, Jiang X, Mehmood K, Zhao X, Li J (2018) Socio-economic burden of parasitic infections in yaks from 1984 to 2017 on Qinghai Tibetan Plateau of China-a review. Acta Trop 183:103–109. https://doi.org/10.1016/j.actatropica.2018.04.011
Wei Y, Wang S, Fang Y, Nawaz Z (2017) Integrated assessment on the vulnerability of animal husbandry to snow disasters under climate change in the Qinghai-Tibetan Plateau. Glob Planet Change 157:139–152
Hao L, Yuan D, Li S, Jia T, Guo L, Hou W, Lu Z, Mo X, Yin J, Yang A, Zheng W, Li R (2020) Detection of Theileria spp. in ticks, sheep keds (Melophagus ovinus), and livestock in the Eastern Tibetan Plateau. China. Parasitol Res 119(8):2641–2648. https://doi.org/10.1007/s00436-020-06757-6
Guo W, Wang W, Bi S, Long R, Ullah F, Shafiq M, Zhou M, Zhang Y (2020) Characterization of anaerobic rumen fungal community composition in yak, Tibetan sheep and small tail han sheep grazing on the Qinghai-Tibetan Plateau. Animals 10(1):144
Ebrahimipour M, Budke CM, Harandi MF (2020) Control of cystic echinococcosis in Iran: where do we stand? Trends Parasitol 36(7):578–581. https://doi.org/10.1016/j.pt.2020.04.007
Han R, Yang JF, Mukhtar MU, Chen Z, Niu QL, Lin YQ, Liu GY, Luo JX, Yin H, Liu ZJ (2019) Molecular detection of Anaplasma infections in ixodid ticks from the Qinghai-Tibet Plateau. Infect Dis Poverty 8(1):12. https://doi.org/10.1186/s40249-019-0522-z
Qin SY, Zhou DH, Cong W, Zhang XX, Lou ZL, Yin MY, Tan QD, Zhu XQ (2015) Seroprevalence, risk factors and genetic characterization of Toxoplasma gondii in free-range white yaks (Bos grunniens) in China. Vet Parasitol 211(3):300–302. https://doi.org/10.1016/j.vetpar.2015.05.015
Wang J, Blasdell KR, Yin H, Walker PJ (2017) A large-scale serological survey of Akabane virus infection in cattle, yak, sheep and goats in China. Vet Microbiol 207:7–12. https://doi.org/10.1016/j.vetmic.2017.05.014
Xulong L, Hailong Q, Zhaoyang B, Yanling Y, Chunhui S, Xiaoyan L, Jinglong W, Jinshan C, Ruilin M, Yijuan F (2011) Seroprevalence of Brucella infection in yaks (Bos grunniens) on the Qinghai-Tibet Plateau of China. Trop Anim Health Prod 43(2):305–306
Wang H, Yang J, Mukhtar MU, Liu Z, Zhang M, Wang X (2019) Molecular detection and identification of tick-borne bacteria and protozoans in goats and wild Siberian roe deer (Capreolus pygargus) from Heilongjiang province, Northeastern China. Parasit Vectors 12(1):296. https://doi.org/10.1186/s13071-019-3553-1
Juan C, ChengCheng L, YaE Z, Li H, YuanJun Y, Fan Y, ZhiYun S (2015) Population identification and divergence threshold in Psoroptidae based on ribosomal ITS2 and mitochondrial COI genes. Parasitol Res 114(9):3497–3507. https://doi.org/10.1007/s00436-015-4578-9
Sun M, Wang J, Liu Z, Guan G, Li Y, Liu J, Xu J, Yin H, Luo J (2019) First molecular evidence of Babesia occultans and Theileria separata infection in ticks and sheep in China. Exp Appl Acarol 78(2):223–229. https://doi.org/10.1007/s10493-019-00369-1
Sun M, Guan G, Liu Z, Wang J, Wang D, Wang S, Ma C, Cheng S, Yin H, Luo J (2020) Molecular survey and genetic diversity of Babesia spp. and Theileria spp. in cattle in Gansu province, China. Acta parasitol 19:1–8
Altay K, Dumanli N, Aktas M (2007) Molecular identification, genetic diversity and distribution of Theileria and Babesia species infecting small ruminants. Vet Parasitol 147(1–2):161–165
Nagore D, Garcıa-Sanmartın J, Garcıa-Pérez AL, Juste RA, Hurtado A (2004) Identification, genetic diversity and prevalence of Theileria and Babesia species in a sheep population from northern Spain. Int J Parasitol 34(9):1059–1067
Agbemabiese CA, Nakagomi T, Damanka SA, Dennis FE, Lartey BL, Armah GE, Nakagomi O (2019) Sub-genotype phylogeny of the non-G, non-P genes of genotype 2 rotavirus A strains. PLoS One 14(5):e0217422. https://doi.org/10.1371/journal.pone.0217422
Ramos V, Morais J, Vasconcelos VM (2017) A curated database of cyanobacterial strains relevant for modern taxonomy and phylogenetic studies. Sci Data 4(1):1–8
Kindu A, Getaneh G (2016) Prevalence of avian tuberculosis in domestic chickens in selected sites of Ethiopia. J Vet Sci Technol 7(377):2
Ko Y-K, Soh M-A, Kang S-H, Lee J-I (2013) The prevalence of metabolic syndrome in schizophrenic patients using antipsychotics. Clin Psychopharmacol Neurosci 11(2):80
Guo WP, Huang B, Zhao Q, Xu G, Liu B, Wang YH, Zhou EM (2018) Human-pathogenic Anaplasma spp., and Rickettsia spp. in animals in Xi’an China. PLoS Negl Trop Dis 12(11):e0006916. https://doi.org/10.1371/journal.pntd.0006916
Riaz M, Nazir MM, Tasawar Z, Ahmed AN, Ayaz MM, Akram Q, Lindsay DS (2019) Molecular epidemiology and prevalence of Theileria lestoquardi and Theileria ovis infection in goats infested with tick vectors from Multan. Pak J Med Entomol 56(3):844–848. https://doi.org/10.1093/jme/tjy229
Li Y, Li X, Liu J, Wang J, Jia D, Liu A, He Z, Guan G, Liu Z, Liu G, Luo J, Yin H (2019) First Report of Theileria Infection of Bactrian Camels (Camelus bactrianus) in Xinjiang China. Acta Parasitol 64(4):923–926. https://doi.org/10.2478/s11686-019-00086-0
Forteau L, Dumont B, Sallé G, Bigot G, Fleurance G (2020) Horses grazing with cattle have reduced strongyle egg count due to the dilution effect and increased reliance on macrocyclic lactones in mixed farms. Animal 14(5):1076–1082. https://doi.org/10.1017/s1751731119002738
Li S, Liu J, Liu A, Li Y, Wang S, Wang S, Yin H, Luo J, Guan G (2017) Molecular investigation of piroplasma infection in white yaks (Bos grunniens) in Gansu province, China. Acta Trop 171:220–225. https://doi.org/10.1016/j.actatropica.2017.04.009
Liu A, Guan G, Liu Z, Liu J, Leblanc N, Li Y, Gao J, Ma M, Niu Q, Ren Q, Bai Q, Yin H, Luo J (2010) Detecting and differentiating Theileria sergenti and Theileria sinensis in cattle and yaks by PCR based on major piroplasm surface protein (MPSP). Exp Parasitol 126(4):476–481. https://doi.org/10.1016/j.exppara.2010.05.024
Sun C, Liu Z, Gao J, Guan G, Ma M, Luo J, Yin H (2008) Investigations into the natural infection rate of Haemaphysalis qinghaiensis with piroplasma using a nested PCR. Exp Appl Acarol 44(2):107–114. https://doi.org/10.1007/s10493-008-9133-2
Chen Z, Yang X, Bu F, Yang X, Yang X, Liu J (2010) Ticks (acari: ixodoidea: argasidae, ixodidae) of China. Exp Appl Acarol 51(4):393–404. https://doi.org/10.1007/s10493-010-9335-2
Teng KF, Jiang ZJ, Teng G, Jiang Z (1991) Economic insect fauna of China, vol 39. Ixodidae. Science Press, Beijing Acari
Hao L, Yuan D, Li S, Jia T, Guo L, Hou W, Lu Z, Mo X, Yin J, Yang A, Zheng W, Li R (2020) Detection of Theileria spp. in ticks, sheep keds (Melophagus ovinus), and livestock in the eastern Tibetan Plateau China. Parasitol Res 119(8):2641–2648. https://doi.org/10.1007/s00436-020-06757-6
Homer MJ, Aguilar-Delfin I, Telford SR 3rd, Krause PJ, Persing DH (2000) Babesiosis. Clin Microbiol Rev 13(3):451–469. https://doi.org/10.1128/cmr.13.3.451-469.2000
Zhao L, Li J, Cui X, Jia N, Wei J, Xia L, Wang H, Zhou Y, Wang Q, Liu X, Yin C, Pan Y, Wen H, Wang Q, Xue F, Sun Y, Jiang J, Li S, Cao W (2020) Distribution of Haemaphysalis longicornis and associated pathogens: analysis of pooled data from a China field survey and global published data. Lancet Planet Health 4(8):e320–e329. https://doi.org/10.1016/s2542-5196(20)30145-5
Gallusová M, Qablan MA, D’Amico G, Oborník M, Petrželková KJ, Mihalca AD, Modrý D (2014) Piroplasms in feral and domestic equines in rural areas of the Danube Delta, Romania, with survey of dogs as a possible reservoir. Vet Parasitol 206(3–4):287–292. https://doi.org/10.1016/j.vetpar.2014.10.018
Zhang YK, Zhang XY, Liu JZ (2019) Ticks (Acari: Ixodoidea) in China: geographical distribution, host diversity, and specificity. Arch Insect Biochem Physiol 102(3):e21544
Yang Y, Mao Y, Kelly P, Yang Z, Luan L, Zhang J, Li J, El-Mahallawy HS, Wang C (2014) A pan-Theileria FRET-qPCR survey for Theileria spp. in ruminants from nine provinces of China. Parasit Vectors 7:413–413. https://doi.org/10.1186/1756-3305-7-413
Rjeibi MR, Darghouth MA, Gharbi M (2016) Prevalence of Theileria and Babesia species in Tunisian sheep. Onderstepoort J Vet Res 83(1):a1040. https://doi.org/10.4102/ojvr.v83i1.1040
Alanazi AD, Said AE, Ghoneim AM, Alyousif MS, Alanazi IO (2019) A comprehensive evaluation and first molecular report of Theileria ovis infection in small ruminants in Saudi Arabia. Trop Anim Health Prod 51(1):89–98. https://doi.org/10.1007/s11250-018-1663-y
Altay K, Dumanli N, Holman PJ, Aktas M (2005) Detection of Theileria ovis in naturally infected sheep by nested PCR. Vet Parasitol 127(2):99–104. https://doi.org/10.1016/j.vetpar.2004.09.012
Zarei F, Ganjali M, Nabavi R (2019) Identification of Theileria species in sheep and vector ticks using pcr method in Zabol. East Iran J Arthropod Borne Dis 13(1):76–82
Gebrekidan H, Hailu A, Kassahun A, Rohoušová I, Maia C, Talmi-Frank D, Warburg A, Baneth G (2014) Theileria infection in domestic ruminants in northern Ethiopia. Vet Parasitol 200(1–2):31–38. https://doi.org/10.1016/j.vetpar.2013.11.017
Li Y, Guan G, Liu A, Peng Y, Luo J, Yin H (2010) Experimental transmission of Theileria ovis by Hyalomma anatolicum anatolicum. Parasitol Res 106(4):991–994. https://doi.org/10.1007/s00436-010-1763-8
Bishop R, Musoke A, Morzaria S, Gardner M, Nene V (2004) Theileria: intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitology 129(Suppl):S271-283. https://doi.org/10.1017/s0031182003004748
Nagore D, García-Sanmartín J, García-Pérez AL, Juste RA, Hurtado A (2004) Identification, genetic diversity and prevalence of Theileria and Babesia species in a sheep population from Northern Spain. Int J Parasitol 34(9):1059–1067. https://doi.org/10.1016/j.ijpara.2004.05.008
Nair AS, Ravindran R, Lakshmanan B, Kumar SS, Tresamol PV, Saseendranath MR, Senthilvel K, Rao JR, Tewari AK, Ghosh S (2011) Haemoprotozoa of cattle in northern Kerala. India Trop Biomed 28(1):68–75
Acknowledgements
This work was supported by the Regular Assistance Project of the International Department of the Ministry of Science and Technology of China (Grant Numbers KY201904013); the Chinese Academy of Sciences (Grant Numbers CZBZX-1); the National Forestry and Grassland Administration, China. We are very grateful for the cooperation of the local herdsmen and the full assistance of the veterinary staff in Yu Shu, Qu Ma Lai, Zhi Duo, Ma Qin, Da Ri, and Ban Ma during the sample collection process.
Funding
This work was supported by the Regular Assistance Project of International Department of the Ministry of Science and Technology of China (Grant Numbers KY201904013); the Chinese Academy of Sciences (Grant Numbers CZBZX-1); the National Forestry and Grassland Administration, China.
Author information
Authors and Affiliations
Contributions
HXH took the lead in designing and performing the study. YW and QXZ designed the study in detail. YW, QXZ and YL performed the sample collection. YW, SLW, ZWY and BW performed the DNA extraction and PCR, YW and BW analyzed the data. BW wrote the manuscript. BW, QXZ, WY, SYH and GHY revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Approval
All animal studies were approved by the Committee on the Ethics of Animal Experiments in the Institute of Zoology, Chinese Academy of Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, B., Zhang, Q. et al. The Common Occurrence of Theileria ovis in Tibetan Sheep and the First Report of Theileria sinensis in Yaks from Southern Qinghai, China. Acta Parasit. 66, 1177–1185 (2021). https://doi.org/10.1007/s11686-021-00381-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-021-00381-9