Abstract
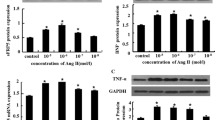
The aim of this study was to explore the regulatory mechanism of retinoic acid (RA) on the TBX1 gene expression in myocardial cells. Ventricular cardiocytes were isolated from neonatal rats and cultured, and then treated with different concentrations of retinoic acid. The expression of Shh and Fgf8 at mRNA and protein levels in neonatal rat myocardial cells were measured by using RT-PCR and Western blot technique, respectively. There was basal expression of Shh and Fgf8 in the control group. When treated with 3 × 10−7 mol/L RA, we observed that the expression of Shh mRNA and protein in neonatal rat myocardial cells were up-regulated by 1.51 (P < 0.05) and 1.10 times (P < 0.05), respectively. In comparison with the control group, under the concentration of 5 × 10−7 mol/L RA, they were up-regulated by 2.21 (P < 0.05) and 2.38 times (P < 0.05) individually. Meanwhile, we could detect that the expression of Fgf8 mRNA and protein were up-regulated by 2.50 times (P < 0.05) and 80% (P < 0.05) separately compared with the control group after stimulation of 3 × 10−7 mol/L RA, and they were up-regulated by 3.48 (P < 0.05) and 2.04 times (P < 0.05) individually after stimulation of 5 × 10−7 mol/L RA. The results indicated that RA could induce the expression of Shh and Fgf8 in neonatal rat myocardial cells. At the same time, it has shown that Shh and Fgf8 were involved in the regulation process of RA on TBX1 expression.
Similar content being viewed by others
References
Ben-Shachar S, Ou Z, Shaw C A, Belmont JW, Patel M S, Hummel M, Amato S, Tartaglia N, Berg J, Sutton V R, Lalani S R, Chinault A C, Cheung S W, Lupski J R, Patel A. 22q11.2 distal deletion: a recurrent genomic disorder distinct from DiGeorge syndrome and velocardiofacial syndrome. Am J Hum Genet, 2008, 82(1): 214–221
Arnold J S, Braunstein E M, Ohyama T, Groves A K, Adams J C, Brown M C, Morrow B E. Tissue-specific roles of TBX1 in the development of the outer, middle and inner ear, defective in 22q11DS patients. Hum Mol Genet, 2006, 15(10): 1629–1639
Vermot J, Messaddeq N, Niederreither K, Dierich A, Dolle P. Rescue of morphogenetic defects and of retinoic acid signaling in retinaldehyde dehydrogenase 2 (Raldh2) mouse mutants by chimerism with wild-type cells. Differentiation, 2006, 74(9): 661–668
Roberts C, Ivins S, Cook A C, Baldini A, Scambler P J. Cyp26 genes a1, b1 and c1 are down-regulated in TBX1 null mice and inhibition of Cyp26 enzyme function produces a phenocopy of DiGeorge Syndrome in the chick. Hum Mol Genet, 2006, 15(23): 3394–3410
Metzqer D, Chambon P. Contribution of targeted conditional somatic mutagenesis to deciphering retinoid X receptor functions and to generating mouse models of human diseases. Handb Exp Pharmacol, 2007, 178(2): 511–524
Arnold J S, Werling U, Braunstein E M, Liao J, Nowotschin S, Edelmann W, Hebert JM, Morrow B E. Inactivation of TBX1 in the pharyngeal endoderm results in 22q11DS malformations. Development, 2006, 133(5): 977–987
Xu H, Morishima M, Wylie J N, Schwartz R J, Bruneau B G, Lindsay E A, Baldini A. TBX1 has a dual role in the morphogenesis of the cardiac outflow tract. Development, 2004, 131(13): 3217–3227
Zhang Z, Huvnh T, Baldini A. Mesodermal expression of TBX1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development, 2006, 133(18): 3587–3595
Aggarwal V S, Liao J, Bondarev A, Schimmang T, Lewandoski M, Locker J, Shanke A, Campione M, Morrow B E. Dissection of TBX1 and Fgf interactions in mouse models of 22q11DS suggests functional redundancy. Hum Mol Genet, 2006, 15(21): 3219–3228
Zhang L, Zhong T, Wang Y, Jiang Q, Song H, Gui Y. TBX1, a DiGeorge syndrome candidate gene, is inhibited by retinoic acid. Int J Dev Biol, 2006, 50(1): 55–61
Yamagishi H, Maeda J, Hu T, Mcanally J, Conway S J, Kume T, Meyers E N, Yamagishi C, Srivastava D. TBX1 is regulated by tissue-specific forkhead proteins through a common Sonic hedgehog-responsive enhancer. Genes Dev, 2003, 17(2): 269–281
Ivins S, Lammerts van Beuren K, Roberts C, James C, Lindsay E, Baldini A, Ataliotis P, Scambler P J. Microarray analysis detects differentially expressed genes in the pharyngeal region of mice lacking TBX1. Dev Biol, 2005, 285(2): 554–569
Moon A M, Guris D L, Seo J H, Li L, Hammond J, Talbot A, Imamoto A. Crkl deficiency disrupts Fgf8 signaling in a mouse model of 22q11 deletion syndromes. Dev Cell, 2006, 10(1): 71–80
Aggarwal V S, Liao J, Bondarev A, Schimmang T, Lewandoski M, Locker J, Shanske A, Campione M, Morrow B E. Dissection of TBX1 and Fgf interactions in mouse models of 22q11DS suggests functional redundancy. Hum Mol Genet, 2006, 15(21): 3219–3228
Zhang Z, Cerrato F, Xu H, Vitelli F, Morishima M, Vincentz J, Furuta Y, Ma L, Martin J F, Baldini A, Lindsay E. TBX1 expression in pharyngeal epithelia is necessary for pharyngeal arch artery development. Development, 2005, 132(23): 5307–5315
Vitelli F, Taddei I, Morishima M, Meyers E N, Lindsay E A, Baldini A. A genetic link between TBX1 and fibroblast growth factor signaling. Development, 2002, 129(19): 4605–4611
Brown C B, Wenning J M, Lu M M, Epstein D J, Meyers E N, Epstein J A. Cre-mediated excision of Fgf8 in the TBX1 expression domain reveals a critical role for Fgf8 in cardiovascular development in the mouse. Dev Biol, 2004, 267(1): 190–202
Roberts C, Ivins S M, James C T, Scambler P J. Retinoic acid downregulates TBX1 expression in vivo and in vitro. Dev Dyn, 2005, 232 (4): 928–938
Duester G. Retinoic acid regulation of the somitogenesis clock. Birth Defects Res C Embryo Today, 2007, 81(2): 84–92
Wahl M B, Deng C, Lewandoski M, Pourquie O. Fgf signaling acts upstream of the NOTCH and WNT signaling pathways to control segmentation clock oscillations in mouse somitogenesis. Development, 2007, 134(22): 4033–4041
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, M., Wu, X., Xu, J. et al. Influence of retinoic acid on TBX1 expression in myocardial cells induced by Shh and Fgf8. Front. Med. China 3, 61–66 (2009). https://doi.org/10.1007/s11684-009-0007-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11684-009-0007-8