Abstract
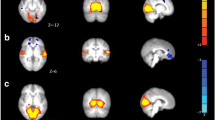
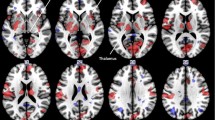
Aberrant functional connectivity of brain networks has been demonstrated in migraine sufferers. Functional magnetic resonance imaging (fMRI) may illustrate altered connectivity in patients suffering from migraine without aura (MwoA). Here, we applied a seed-based approach based on limbic regions to investigate disrupted functional connectivity between spontaneous migraine attacks. Resting-state fMRI data were obtained from 28 migraine patients without aura and 23 well-matched healthy controls (HC). The functional connectivity of the limbic system was characterized using a seed-based whole-brain correlation method. The resulting functional connectivity measurements were assessed for correlations with other clinical features. Neuropsychological data revealed significantly increased connectivity between the limbic system (bilateral amygdala and right hippocampus) and left middle occipital gyrus (MOG), and a positive correlation was revealed between disease duration and connective intensity of the left amygdala and the ipsilateral MOG. There was decreased functional connectivity between the right amygdala and contralateral orbitofrontal cortex (OFC). In addition, resting-state fMRI showed that, compared to HC, patients without aura had significant functional connectivity consolidation between the bilateral hippocampus and cerebellum, and a negative correlation was detected between scores on the headache impact test (HIT) and connectivity intensity of the right hippocampus and bilateral cerebellum. There was decreased functional connectivity between the left hippocampus and three brain areas, encompassing the bilateral inferior parietal gyri (IPG) and contralateral supplementary motor area (SMA). There were no structural differences between the two groups. Our data suggest that migraine patients have disrupted limbic functional connectivity to pain-related regions of the modulatory and encoding cortices, which are associated with specific clinical characteristics. Disturbances of resting-state functional connectivity may play a key role in neuropathological features, perception and affection of migraine. The current study provides further insights into the complex scenario of migraine mechanisms.



Similar content being viewed by others
References
Akerman, S., Romero-Reyes, M., & Holland, P. R. (2017). Current and novel insights into the neurophysiology of migraine and its implications for therapeutics. Pharmacology & Therapeutics, 172, 151–170.
Amin, F. M., Hougaard, A., Magon, S., Sprenger, T., Wolfram, F., Rostrup, E., & Ashina, M. (2018). Altered thalamic connectivity during spontaneous attacks of migraine without aura: a resting-state fMRI study. Cephalalgia, 38, 1237–1244.
Borgdorff, P. (2018). Arguments against the role of cortical spreading depression in migraine. Neurological Research, 40, 173–181.
Boujut, A., & Clarys, D. (2018). Remembering - but not knowing - disturbs the relational bindings newly established in short-term/working memory: an age-group comparison. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 25, 783–802.
Charles, A. (2018). The pathophysiology of migraine: implications for clinical management. The Lancet Neurology, 17, 174–182.
Chen, Y., Xia, W., Chen, H., Feng, Y., Xu, J., Gu, J., Salvi, R., & Yin, X. (2017a). Tinnitus distress is linked to enhanced resting-state functional connectivity from the limbic system to the auditory cortex. Human Brain Mapping, 38, 2384–2397.
Chen, Z., Chen, X., Liu, M., Dong, Z., Ma, L., & Yu, S. (2017b). Altered functional connectivity of amygdala underlying the neuromechanism of migraine pathogenesis. The Journal of Headache and Pain, 18, 7.
Dodick, D. W. (2018). A phase-by-phase review of migraine pathophysiology. Headache: The Journal of Head and Face Pain, 58, 4–16.
Eck, J., Richter, M., Straube, T., Miltner, W. H. R., & Weiss, T. (2011). Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain, 152, 1104–1113.
Ettlin, D. A. (2013). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia, 33, 629–808.
Good, C. D., Johnsrude, I. S., Ashburner, J., Henson, R. N., Friston, K. J., & Frackowiak, R. S. (2001). A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage, 14, 21–36.
Hadjikhani, N., Ward, N., Boshyan, J., Napadow, V., Maeda, Y., Truini, A., Caramia, F., Tinelli, E., & Mainero, C. (2013). The missing link: Enhanced functional connectivity between amygdala and visceroceptive cortex in migraine. Cephalalgia, 33, 1264–1268.
Herman, J. P., Ostrander, M. M., Mueller, N. K., & Figueiredo, H. (2005). Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 29, 1201–1213.
Hertzog, C., Fulton, E. K., Sinclair, S. M., & Dunlosky, J. (2014). Recalled aspects of original encoding strategies influence episodic feelings of knowing. Memory & Cognition, 42, 126–140.
Howard, J. D., & Kahnt, T. (2017). Identity-specific reward representations in orbitofrontal cortex are modulated by selective devaluation. The Journal of Neuroscience, 37, 2627–2638.
Jin, C., Yuan, K., Zhao, L., Zhao, L., Yu, D., von Deneen, K. M., Zhang, M., Qin, W., Sun, W., & Tian, J. (2013). Structural and functional abnormalities in migraine patients without aura. NMR in Biomedicine, 26, 58–64.
Johansen-Berg, H., Behrens, T. E., Robson, M. D., Drobnjak, I., Rushworth, M. F., Brady, J. M., Smith, S. M., Higham, D. J., & Matthews, P. M. (2004). Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 101, 13335–13340.
Kim, J. H., Suh, S., Seol, H. Y., Oh, K., Seo, W., Yu, S., Park, K., & Koh, S. (2008). Regional Grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia, 28, 598–604.
Kosinski, M., Bayliss, M. S., Bjorner, J. B., Ware, J. J., Garber, W. H., Batenhorst, A., Cady, R., Dahlof, C. G., Dowson, A., & Tepper, S. (2003). A six-item short-form survey for measuring headache impact: the HIT-6. Quality of Life Research, 12, 963–974.
Lee, S. H., Kang, Y., & Cho, S. (2017). Subjective cognitive decline in patients with migraine and its relationship with depression, anxiety, and sleep quality. The Journal of Headache and Pain, 18, 77.
Li, K., Zhang, Y., Ning, Y., Zhang, H., Liu, H., Fu, C., Ren, Y., & Zou, Y. (2015). The effects of acupuncture treatment on the right frontoparietal network in migraine without aura patients. The Journal of Headache and Pain, 16:518.
Lima, C. F., Krishnan, S., & Scott, S. K. (2016). Roles of supplementary motor areas in auditory processing and auditory imagery. Trends in Neurosciences, 39, 527–542.
Liu, M., & Chen, J. (2009). Roles of the hippocampal formation in pain information processing. Neuroscience Bulletin, 25, 237–266.
Liu, J., Lan, L., Li, G., Yan, X., Nan, J., Xiong, S., Yin, Q., von Deneen, K. M., Gong, Q., Liang, F., Qin, W., & Tian, J. (2013). Migraine-related gray matter and white matter changes at a 1-year follow-up evaluation. The Journal of Pain, 14, 1703–1708.
Liu, J., Lan, L., Mu, J., Zhao, L., Yuan, K., Zhang, Y., Huang, L., Liang, F., & Tian, J. (2015). Genetic contribution of catechol-O-methyltransferase in hippocampal structural and functional changes of female migraine sufferers. Human Brain Mapping, 36, 1782–1795.
Liu, H., Chou, K., Lee, P., Fuh, J., Niddam, D. M., Lai, K., Hsiao, F., Lin, Y., Chen, W., Wang, S., & Lin, C. (2016). Hippocampus and amygdala volume in relation to migraine frequency and prognosis. Cephalalgia, 37, 1329–1336.
Maizels, M., Aurora, S., & Heinricher, M. (2012). Beyond neurovascular: migraine as a dysfunctional Neurolimbic pain network. Headache: The Journal of Head and Face Pain, 52, 1553–1565.
Maleki, N., Becerra, L., Brawn, J., Bigal, M., Burstein, R., & Borsook, D. (2012). Concurrent functional and structural cortical alterations in migraine. CEPHALALGIA, 32, 607–620.
Maleki, N., Becerra, L., Brawn, J., McEwen, B., Burstein, R., & Borsook, D. (2013). Common hippocampal structural and functional changes in migraine. Brain Structure and Function, 218, 903–912.
Mendonca-de-Souza, M., Monteiro, U. M., Bezerra, A. S., Silva-de-Oliveira, A. P., Ventura-da-Silva, B. R., Barbosa, M. S., de Souza, J. A., Criado, E. C., Ferrarezi, M. C., Alencar, G. A., Lins, O. G., Coriolano, M., Costa, B. L., & Rodrigues, M. C. (2012). Resilience in migraine brains: decrease of coherence after photic stimulation. Frontiers in Human Neuroscience, 6, 207.
Moulton, E. A., Becerra, L., Maleki, N., Pendse, G., Tully, S., Hargreaves, R., Burstein, R., & Borsook, D. (2011). Painful heat reveals Hyperexcitability of the temporal pole in Interictal and ictal migraine states. Cerebral Cortex, 21, 435–448.
Mueller, S., Keeser, D., Samson, A. C., Kirsch, V., Blautzik, J., Grothe, M., Erat, O., Hegenloh, M., Coates, U., Reiser, M. F., Hennig-Fast, K., & Meindl, T. (2013). Convergent findings of altered functional and structural brain connectivity in individuals with high functioning autism: a multimodal MRI study. PLoS ONE, 8, e67329.
Nachev, P., Kennard, C., & Husain, M. (2008). Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews. Neuroscience, 9, 856–869.
Neubert, F. X., Mars, R. B., Sallet, J., & Rushworth, M. F. (2015). Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 112, E2695–E2704.
Ning, Y., Zheng, R., Li, K., Zhang, Y., Lyu, D., Jia, H., Ren, Y., & Zou, Y. (2018). The altered granger causality connection among pain-related brain networks in migraine. Medicine, 97, e102.
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia, 9, 97–113.
Peyron, R., Garcia-Larrea, L., Gregoire, M. C., Convers, P., Richard, A., Lavenne, F., Barral, F. G., Mauguiere, F., Michel, D., & Laurent, B. (2000). Parietal and cingulate processes in central pain. A combined positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) study of an unusual case. Pain, 84, 77–87.
Rocca, M. A., Ceccarelli, A., Falini, A., Colombo, B., Tortorella, P., Bernasconi, L., Comi, G., Scotti, G., & Filippi, M. (2006). Brain gray matter changes in migraine patients with T2-visible lesions. STROKE, 37, 1765–1770.
Rudebeck, P. H., & Rich, E. L. (2018). Orbitofrontal cortex. Current Biology, 28, R1083–R1088.
Russo, A., Tessitore, A., Esposito, F., Marcuccio, L., Giordano, A., Conforti, R., Truini, A., Paccone, A., D Onofrio, F., & Tedeschi, G. (2012a). Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. Journal of Neurology, 259, 1903–1912.
Russo, A., Tessitore, A., Giordano, A., Corbo, D., Marcuccio, L., De Stefano, M., Salemi, F., Conforti, R., Esposito, F., & Tedeschi, G. (2012b). Executive resting-state network connectivity in migraine without aura. Cephalalgia, 32, 1041–1048.
Santangelo, G., Russo, A., Tessitore, A., Garramone, F., Silvestro, M., Della, M. M., Marcuccio, L., Fornaro, I., Trojano, L., & Tedeschi, G. (2018). Prospective memory is dysfunctional in migraine without aura. Cephalalgia, 38, 1825–1832.
Schwedt, T. J., & Dodick, D. W. (2009). Advanced neuroimaging of migraine. The Lancet Neurology, 8, 560–568.
Schwedt, T. J., Chong, C. D., Chiang, C., Baxter, L., Schlaggar, B. L., & Dodick, D. W. (2014). Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. CEPHALALGIA, 34, 947–958.
Schwedt, T. J., Chiang, C. C., Chong, C. D., & Dodick, D. W. (2015). Functional MRI of migraine. Lancet Neurology, 14, 81–91.
Seo, J. G., & Park, S. P. (2015a). Validation of the generalized anxiety disorder-7 (GAD-7) and GAD-2 in patients with migraine. The Journal of Headache and Pain, 16, 97.
Seo, J. G., & Park, S. P. (2015b). Validation of the patient health Questionnaire-9 (PHQ-9) and PHQ-2 in patients with migraine. The Journal of Headache and Pain, 16, 65.
Spitzer, R. L., Kroenke, K., Williams, J. B., & Lowe, B. (2006). A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine, 166, 1092–1097.
Stankewitz, A., & May, A. (2011). Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology, 77, 476–482.
Szabó, N., Faragó, P., Király, A., Veréb, D., Csete, G., Tóth, E., Kocsis, K., Kincses, B., Tuka, B., Párdutz, Á., Szok, D., Tajti, J., Vécsei, L., & Kincses, Z. T. (2018). Evidence for plastic processes in migraine with Aura: a diffusion weighted MRI study. Frontiers in Neuroanatomy, 11:138.
Tso, A. R., Trujillo, A., Guo, C. C., Goadsby, P. J., & Seeley, W. W. (2015). The anterior insula shows heightened interictal intrinsic connectivity in migraine without aura. Neurology, 84, 1043–1050.
Vergani, F., Lacerda, L., Martino, J., Attems, J., Morris, C., Mitchell, P., Thiebaut, D. S. M., & Dell'Acqua, F. (2014). White matter connections of the supplementary motor area in humans. Journal of Neurology, Neurosurgery, and Psychiatry, 85, 1377–1385.
Vincent, J. L., Snyder, A. Z., Fox, M. D., Shannon, B. J., Andrews, J. R., Raichle, M. E., & Buckner, R. L. (2006). Coherent spontaneous activity identifies a hippocampal-parietal memory network. Journal of Neurophysiology, 96, 3517–3531.
Wang, J. J., Chen, X., Sah, S. K., Zeng, C., Li, Y. M., Li, N., Liu, M. Q., & Du, S. L. (2016). Amplitude of low-frequency fluctuation (ALFF) and fractional ALFF in migraine patients: a resting-state functional MRI study. Clinical Radiology, 71, 558–564.
Wang, M., Su, J., Zhang, J., Zhao, Y., Yao, Q., Zhang, Q., Zhang, H., Wang, S., Li, G., Liu, J., & Du, X. (2017). Visual cortex and cerebellum hyperactivation during negative emotion picture stimuli in migraine patients. SCI REP-UK, 7:41919.
Wewers, M. E., & Lowe, N. K. (1990). A critical review of visual analogue scales in the measurement of clinical phenomena. Research in Nursing & Health, 13, 227–236.
Wilcox, S. L., Veggeberg, R., Lemme, J., Hodkinson, D. J., Scrivani, S., Burstein, R., Becerra, L., & Borsook, D. (2016). Increased functional activation of limbic brain regions during negative emotional processing in migraine. Frontiers in Human Neuroscience, 10:366.
Wise, S. P., Boussaoud, D., Johnson, P. B., & Caminiti, R. (1997). premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annual Review of Neuroscience, 20, 25–42.
Yang, M., Yang, J., Zeng, F., Liu, P., Lai, Z., Deng, S., Fang, L., Song, W., Xie, H., & Liang, F. (2014). Electroacupuncture stimulation at sub-specific acupoint and non-acupoint induced distinct brain glucose metabolism change in migraineurs: a PET-CT study. Journal of Translational Medicine, 12, 351.
Yu, D., Yuan, K., Zhao, L., Zhao, L., Dong, M., Liu, P., Wang, G., Liu, J., Sun, J., Zhou, G., Deneen, K. M., Liang, F., Qin, W., & Tian, J. (2012). Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting-state study. NMR in Biomedicine, 25, 806–812.
Yu, D., Yuan, K., Luo, L., Zhai, J., Bi, Y., Xue, T., Ren, X., Zhang, M., Ren, G., & Lu, X. (2017). Abnormal functional integration across core brain networks in migraine without aura. Molecular Pain, 13, 1940341042.
Zhang, J., Su, J., Wang, M., Zhao, Y., Yao, Q., Zhang, Q., Lu, H., Zhang, H., Wang, S., Li, G., Wu, Y., Liu, F., Shi, Y., Li, J., Liu, J., & Du, X. (2016). Increased default mode network connectivity and increased regional homogeneity in migraineurs without aura. The Journal of Headache and Pain, 17, 98.
Zhao, L., Liu, J., Dong, X., Peng, Y., Yuan, K., Wu, F., Sun, J., Gong, Q., Qin, W., & Liang, F. (2013). Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. The Journal of Headache and Pain, 14, 1–9.
Acknowledgements
We would like to thank all the migraine patients and healthy controls who participated in our research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wei, HL., Chen, J., Chen, YC. et al. Impaired functional connectivity of limbic system in migraine without aura. Brain Imaging and Behavior 14, 1805–1814 (2020). https://doi.org/10.1007/s11682-019-00116-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-019-00116-5