Abstract
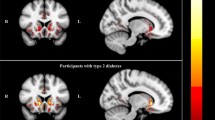
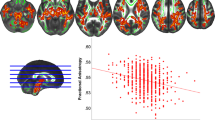
Type 1 diabetes is associated with slower psychomotor speed, but the neural basis of this relationship is not yet understood. The basal ganglia are a set of structures that are vulnerable to small vessel disease, particularly in individuals with type 1 diabetes. Thus, we examined the relationship between psychomotor speed and resting state resting cerebral blood flow in a sample of adults with diabetes onset during childhood (≤ 17 years of age). The sample included 77 patients (39 M, 38 F) with a mean age of 47.43 ± 5.72 years, age of onset at 8.50 ± 4.26 years, and duration of disease of 38.92 ± 4.18 years. Resting cerebral blood flow was quantified using arterial spin labeling. After covarying for sex, years of education and normalized gray matter volume, slower psychomotor speed was associated with lower cerebral blood flow in bilateral caudate nucleus-thalamus and a region in the superior frontal gyrus. These results suggest that the basal ganglia and frontal cortex may underlie slower psychomotor speed in individuals with type 1 diabetes.


Similar content being viewed by others
References
Alsop, D. C., Detre, J. A., Golay, X., Günther, M., Hendrikse, J., Hernandez-Garcia, L., … Zaharchuk, G. (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine, 73(1), spcone. https://doi.org/10.1002/mrm.25607.
Batista, S., Zivadinov, R., Hoogs, M., Bergsland, N., Heininen-Brown, M., Dwyer, M. G., … Benedict, R. H. B. (2012). Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. Journal of Neurology, 259(1), 139–146. https://doi.org/10.1007/s00415-011-6147-1.
Bernbaum, M., Menon, B. K., Fick, G., Smith, E. E., Goyal, M., Frayne, R., & Coutts, S. B. (2015). Reduced blood flow in normal white matter predicts development of leukoaraiosis. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism, 35(10), 1610–1615. https://doi.org/10.1038/jcbfm.2015.92.
Bolo, N. R., Musen, G., Simonson, D. C., Nickerson, L. D., Flores, V. L., Siracusa, T., … Jacobson, A. M. (2015a). Functional connectivity of insula, basal ganglia, and prefrontal executive control networks during hypoglycemia in type 1 diabetes. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(31), 11012–11023. https://doi.org/10.1523/JNEUROSCI.0319-15.2015.
Bolo, N. R., Musen, G., Simonson, D. C., Nickerson, L. D., Flores, V. L., Siracusa, T., … Jacobson, A. M. (2015b). Functional connectivity of insula, basal ganglia, and prefrontal executive control networks during hypoglycemia in type 1 diabetes. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35(31), 11012–11023. https://doi.org/10.1523/JNEUROSCI.0319-15.2015.
Cranston, I., Reed, L. J., Marsden, P. K., & Amiel, S. A. (2001). Changes in regional brain (18)F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counter-regulatory failure. Diabetes, 50(10), 2329–2336.
Detre, J. A., Rao, H., Wang, D. J. J., Chen, Y. F., & Wang, Z. (2012). Applications of arterial spin labeled MRI in the brain. Journal of Magnetic Resonance Imaging: JMRI, 35(5), 1026–1037. https://doi.org/10.1002/jmri.23581.
Detre J. A., & Wang, J. (2002). Technical aspects and utility of fMRI using BOLD and ASL. Clinical Neurophysiology, 113, 621–634. https://doi.org/10.1016/S1388-2457(02)00038-X.
Duckrow, R. B. (1995). Decreased cerebral blood flow during acute hyperglycemia. Brain Research, 703(1–2), 145–150.
Feil, D. G., Zhu, C. W., & Sultzer, D. L. (2012). The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with Type 2 diabetes. Journal of Behavioral Medicine, 35(2), 190–199. https://doi.org/10.1007/s10865-011-9344-6.
Gallardo-Moreno, G. B., González-Garrido, A. A., Gudayol-Ferré, E., & Guàrdia-Olmos, J. (2015). Type 1 diabetes modifies brain activation in young patients while performing visuospatial working memory tasks. Journal of Diabetes Research, 2015, 703512. https://doi.org/10.1155/2015/703512.
Graybiel, A. M., Aosaki, T., Flaherty, A. W., & Kimura, M. (1994). The basal ganglia and adaptive motor control. Science (New York), 265(5180), pp. 1826–1831.
Hays, C. C., Zlatar, Z. Z., & Wierenga, C. E. (2016). The utility of cerebral blood flow as a biomarker of preclinical Alzheimer’s disease. Cellular and Molecular Neurobiology. https://doi.org/10.1007/s10571-015-0261-z.
Heikkilä, O., Lundbom, N., Timonen, M., Groop, P.-H., Heikkinen, S., & Mäkimattila, S. (2010). Evidence for abnormal glucose uptake or metabolism in thalamus during acute hyperglycaemia in type 1 diabetes—a 1H MRS study. Metabolic Brain Disease, 25(2), 227–234. https://doi.org/10.1007/s11011-010-9199-5.
Hughes, T. M., Ryan, C. M., Aizenstein, H. J., Nunley, K., Gianaros, P. J., Miller, R., … Rosano, C. (2013). Frontal gray matter atrophy in middle aged adults with type 1 diabetes is independent of cardiovascular risk factors and diabetes complications. Journal of Diabetes and Its Complications, 27(6), 558–564. https://doi.org/10.1016/j.jdiacomp.2013.07.001.
Hwang, M., Tudorascu, D. L., Nunley, K., Karim, H., Aizenstein, H. J., Orchard, T. J., & Rosano, C. (2016). Brain activation and psychomotor speed in middle-aged patients with type 1 diabetes: relationships with hyperglycemia and brain small vessel disease. Journal of Diabetes Research, 2016, 9571464. https://doi.org/10.1155/2016/9571464.
Jacobs, H. I. L., Leritz, E. C., Williams, V. J., Van Boxtel, M. P. J., van der Elst, W., Jolles, J., … Salat, D. H. (2013). Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Human Brain Mapping, 34(1), 77–95. https://doi.org/10.1002/hbm.21412.
Jiménez-Bonilla, J. F., Quirce, R., Hernández, A., Vallina, N. K., Guede, C., Banzo, I., … Carril, J. M. (2001). Assessment of cerebral perfusion and cerebrovascular reserve in insulin-dependent diabetic patients without central neurological symptoms by means of 99mTc-HMPAO SPET with acetazolamide. European Journal of Nuclear Medicine, 28(11), 1647–1655. https://doi.org/10.1007/s002590100595.
Jokinen, P., Karrasch, M., Brück, A., Johansson, J., Bergman, J., & Rinne, J. O. (2013). Cognitive slowing in Parkinson’s disease is related to frontostriatal dopaminergic dysfunction. Journal of the Neurological Sciences, 329(1–2), 23–28. https://doi.org/10.1016/j.jns.2013.03.006.
Kikano, G. E., LaManna, J. C., & Harik, S. I. (1989). Brain perfusion in acute and chronic hyperglycemia in rats. Stroke; a Journal of Cerebral Circulation, 20(8), 1027–1031.
Miller, R. G., Secrest, A. M., Sharma, R. K., Songer, T. J., & Orchard, T. J. (2012). Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes, 61(11), 2987–2992. https://doi.org/10.2337/db11-1625.
Mogenson, G. J., Jones, D. L., & Yim, C. Y. (1980). From motivation to action: functional interface between the limbic system and the motor system. Progress in Neurobiology, 14(2–3), 69–97.
Moulton, C. D., Costafreda, S. G., Horton, P., Ismail, K., & Fu, C. H. Y. (2015). Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging and Behavior, 9(4), 651–662. https://doi.org/10.1007/s11682-014-9348-2.
Naismith, S., Hickie, I., Ward, P. B., Turner, K., Scott, E., Little, C., … Parker, G. (2002). Caudate nucleus volumes and genetic determinants of homocysteine metabolism in the prediction of psychomotor speed in older persons with depression. The American Journal of Psychiatry, 159(12), 2096–2098. https://doi.org/10.1176/appi.ajp.159.12.2096.
Northam, E. A., Rankins, D., Lin, A., Wellard, R. M., Pell, G. S., Finch, S. J., … Cameron, F. J. (2009). Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care, 32(3), 445–450. https://doi.org/10.2337/dc08-1657.
Nunley, K. A., Rosano, C., Ryan, C. M., Jennings, J. R., Aizenstein, H. J., Zgibor, J. C., … Saxton, J. A. (2015). Clinically relevant cognitive impairment in middle-aged adults with childhood-onset type 1 diabetes. Diabetes Care, 38(9), 1768–1776. https://doi.org/10.2337/dc15-0041.
Nunley, K. A., Ryan, C. M., Aizenstein, H. J., MacCloud, R. L., Orchard, T. J., & Rosano, C. (2017). Regional gray matter volumes as related to psychomotor slowing in adults with type 1 diabetes. Psychosomatic Medicine. https://doi.org/10.1097/PSY.0000000000000449.
Nunley, K. A., Ryan, C. M., Orchard, T. J., Aizenstein, H. J., Jennings, J. R., Ryan, J., … Rosano, C. (2015). White matter hyperintensities in middle-aged adults with childhood-onset type 1 diabetes. Neurology, 84(20), 2062–2069. https://doi.org/10.1212/WNL.0000000000001582.
O’Brien, L. M., Ziegler, D. A., Deutsch, C. K., Frazier, J. A., Herbert, M. R., & Locascio, J. J. (2011). Statistical adjustments for brain size in volumetric neuroimaging studies: some practical implications in methods. Psychiatry Research, 193(2), 113–122. https://doi.org/10.1016/j.pscychresns.2011.01.007.
Pambianco, G., Costacou, T., Ellis, D., Becker, D. J., Klein, R., & Orchard, T. J. (2006). The 30-year natural history of type 1 diabetes complications. Diabetes, 55(5), 1463–1469. https://doi.org/10.2337/db05-1423.
Pantoni, L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. The Lancet Neurology, 9(7), 689–701. https://doi.org/10.1016/S1474-4422(10)70104-6.
Pell, G. S., Lin, A., Wellard, R. M., Werther, G. A., Cameron, F. J., Finch, S. J., … Northam, E. A. (2012). Age-related loss of brain volume and T2 relaxation time in youth with type 1 diabetes. Diabetes Care, 35(3), 513–519. https://doi.org/10.2337/dc11-1290.
Quirce, R., Carril, J. M., Jiménez-Bonilla, J. F., Amado, J. A., Gutiérrez-Mendiguchía, C., Banzo, I., … Montero, A. (1997). Semi-quantitative assessment of cerebral blood flow with 99mTc-HMPAO SPET in type I diabetic patients with no clinical history of cerebrovascular disease. European Journal of Nuclear Medicine, 24(12), 1507–1513.
Rooijackers, H. M. M., Wiegers, E. C., Tack, C. J., van der Graaf, M., & de Galan, B. E. (2016). Brain glucose metabolism during hypoglycemia in type 1 diabetes: insights from functional and metabolic neuroimaging studies. Cellular and Molecular Life Sciences: CMLS, 73(4), 705–722. https://doi.org/10.1007/s00018-015-2079-8.
Ryan, C. M., Geckle, M. O., & Orchard, T. J. (2003). Cognitive efficiency declines over time in adults with Type 1 diabetes: effects of micro- and macrovascular complications. Diabetologia, 46(7), 940–948. https://doi.org/10.1007/s00125-003-1128-2.
Seaquist, E. R. (2015). The impact of diabetes on cerebral structure and function. Psychosomatic Medicine, 77(6), 616–621. https://doi.org/10.1097/PSY.0000000000000207.
Selvarajah, D., Wilkinson, I. D., Gandhi, R., Griffiths, P. D., & Tesfaye, S. (2011). Microvascular perfusion abnormalities of the Thalamus in painful but not painless diabetic polyneuropathy: a clue to the pathogenesis of pain in type 1 diabetes. Diabetes Care, 34(3), 718–720. https://doi.org/10.2337/dc10-1550.
Tagougui, S., Fontaine, P., Leclair, E., Aucouturier, J., Matran, R., Oussaidene, K., … Heyman, E. (2015). Regional cerebral hemodynamic response to incremental exercise is blunted in poorly controlled patients with uncomplicated type 1 diabetes. Diabetes Care, 38(5), 858–867. https://doi.org/10.2337/dc14-1792.
Terada, S., Sato, S., Nagao, S., Ikeda, C., Shindo, A., Hayashi, S., … Uchitomi, Y. (2013). Trail making test B and brain perfusion imaging in mild cognitive impairment and mild Alzheimer’s disease. Psychiatry Research, 213(3), 249–255. https://doi.org/10.1016/j.pscychresns.2013.03.006.
van Golen, L. W., Kuijer, J. P. A., Huisman, M. C., Ijzerman, R. G., Barkhof, F., Diamant, M., & Lammertsma, A. A. (2013). Quantification of cerebral blood flow in healthy volunteers and type 1 diabetic patients: Comparison of MRI arterial spin labeling and [(15) O]H2 O positron emission tomography (PET). Journal of Magnetic Resonance Imaging: JMRI. https://doi.org/10.1002/jmri.24484.
Wagener, D. K., Sacks, J. M., LaPorte, R. E., & MaCgregor, J. M. (1982). The Pittsburgh study of insulin-dependent diabetes mellitus: risk for diabetes among relatives of IDDM. Diabetes, 31(2), 136–144. https://doi.org/10.2337/diab.31.2.136.
Wang, J., Aguirre, G. K., Kimberg, D. Y., Roc, A. C., Li, L., & Detre, J. A. (2003). Arterial spin labeling perfusion fMRI with very low task frequency. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine, 49(5), 796–802. https://doi.org/10.1002/mrm.10437.
Wolk, D. A., & Detre, J. A. (2012). Arterial spin labeling MRI: an emerging biomarker for Alzheimer’s disease and other neurodegenerative conditions. Current Opinion in Neurology, 25(4), 421–428. https://doi.org/10.1097/WCO.0b013e328354ff0a.
Wong, R. H. X., Scholey, A., & Howe, P. R. C. (2014). Assessing premorbid cognitive ability in adults with type 2 diabetes mellitus–a review with implications for future intervention studies. Current Diabetes Reports, 14(11), 547. https://doi.org/10.1007/s11892-014-0547-4.
Wright, S. N., Hong, L. E., Winkler, A. M., Chiappelli, J., Nugent, K., Muellerklein, F., … Kochunov, P. (2015). Perfusion shift from white to gray matter may account for processing speed deficits in schizophrenia. Human Brain Mapping, 36(10), 3793–3804. https://doi.org/10.1002/hbm.22878.
Wu, M., Rosano, C., Butters, M., Whyte, E., Nable, M., Crooks, R., … Aizenstein, H. J. (2006). A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Research, 148(2–3), 133–142. https://doi.org/10.1016/j.pscychresns.2006.09.003.
Acknowledgements
The authors wish to acknowledge Dr. Christopher Ryan and Dr. Judith Saxton for their assistance in the development and administration of the neuropsychology protocol.
Funding
This study was funded by National Institutes of Health grants DK095759 (Ryan), AG037451 (Rosano), DK089028 (Rosano), AG024827 (Rosano), DK034818 (Orchard) and the Rossi Memorial Fund (Orchard).
Author information
Authors and Affiliations
Contributions
JPR analyzed the data and wrote the manuscript; HJA oversaw data analysis and processing; TJO is responsible for recruitment and maintenance of the cohort; KAN oversaw data collection, management and assisted in manuscript preparation; HK performed data analyses and assisted in manuscript preparation; CR designed the study, oversaw data collection/management, and assisted in manuscript preparation.
Corresponding author
Ethics declarations
All participants provided written informed consent prior to study participation. The University of Pittsburgh Institutional Review Board approved the study.
Sources of support
DK095759 (Ryan), AG037451 (Rosano), DK089028 (Rosano), AG024827 (Rosano), DK034818 (Orchard), and the Rossi Memorial Fund (Orchard).
Conflict of interest
The authors have no conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ryan, J.P., Aizenstein, H.J., Orchard, T.J. et al. Basal ganglia cerebral blood flow associates with psychomotor speed in adults with type 1 diabetes. Brain Imaging and Behavior 12, 1271–1278 (2018). https://doi.org/10.1007/s11682-017-9783-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11682-017-9783-y