Abstract
Phase relations in the system Cu-Eu-O have been determined by equilibrating samples of different average composition at 1200 K and by phase analysis after quenching using optical microscopy (OM), x-ray diffraction (XRD), scanning electron microscopy (SEM), and energy dispersive x-ray (EDX). The equilibration experiments were conducted in evacuated ampoules and under flowing inert gas and pure oxygen. The Cu-Eu alloys were found to be in equilibrium with EuO. The higher oxides of europium, Eu3O4 and Eu2O3, coexist with metallic copper. Two ternary oxides CuEu2O4 and CuEuO2 were found to be stable. The ternary oxide CuEuO2, with copper in the monovalent state, can coexist with Cu, Cu2O, Eu2O3 and CuEu2O4 in different phase fields. The compound CuEu2O4 can be in equilibrium with Cu2O, CuO, CuEuO2, Eu2O3, and O2 gas under different conditions at 1200 K. Thermodynamic properties of the ternary oxides were determined using three solid-state cells based on yttria-stabilized zirconia as the electrolyte in the temperature range from 875 to 1250 K. The cells essentially measure the oxygen chemical potential in the three-phase fields: Cu+Eu2O3+CuEuO2, Cu2O+CuEuO2+CuEu2O4, and Eu2O3+CuEuO2+CuEu2O4. The thermodynamic properties of the ternary oxides can be represented by the equations:
Thermogravimetric analysis (TGA) studies in Ar+O2 mixtures confirmed the results from emf measurements. An oxygen potential diagram for the system Cu-Eu-O at 1200 K was evaluated from the results of this study and information available in the literature on the binary phases.
Similar content being viewed by others
References
Yu.D. Tretyakov, A.R. Kaul, and N.V. Makukhin: “An Electrochemical Study of High-Temperature Stability of Compounds Between the Rare Earths and Copper Oxide,” J. Solid State Chem., 1976, 17, pp. 183–89.
Y. Idemoto, I. Oyagi, and K. Fueki: “Determination of Thermodynamic Data of Ln1.85Ce0.15CuO4 and Ln2CuO4 (Ln=Nd. Sm. Eu) by the EMF Method,” Physica C, 1992, 195, pp. 269–76.
A.N. Petrov, A.Yu. Zuev, and V.A. Cherepanov: “Thermodynamic Stability of the Lanthanide Cuprites, Ln 2CuO4 and LnCuO2, Where Ln=La, Pr, Nd, Sm, Eu or Gd,” Russ. J. Phys. Chem., 1988, 62, pp. 1613–15.
K. Fitzner: “Gibbs Free Energy of Formation of Solid Phase CuEu2O4 and CuEuO2,” Thermochim. Acta, 1990, 171, pp. 123–30.
E.T. Muromachi and A. Navrotsky: “Thermochemical Study of Ln2O3, T-Ln2CuO4, and Ln2Cu2O5 (Ln=Rare Earth),” J. Solid State Chem., 1993, 106, pp. 349–56.
Y. Idemoto, K. Shizuka, and K. Fueki: “Calorimetric Measurement on Standard Enthalpies of Formation of Ln1.85Ce0.15CuO4 (Ln=Nd, Sm, Eu and Gd) and Ln2CuO4,” Physica C, 1992, 199, pp. 184–90.
J. Foultier, P. Fabry, and M. Kleitz: “Electrochemical Semipermeability and the Electrode Microsystem in Solid Oxide Electrolyte Cells,” J. Electrochem. Soc., 1976, 123, pp. 204–13.
K.T. Jacob and T. Mathews: “Solid State Electrochemical Cells in Process Control,” Ind. J. Tech., 1990, 28, pp. 413–27.
K.T. Jacob and K.P. Jayadevan: “Measurement of Gibbs Energy of Formation of Ca2PbO4 Using a Solid-State Cell With Three Electrodes,” J. Mater. Chem., 1997, 7, pp. 2407–13.
K.T. Jacob and K.P. Jayadevan: “Phase Relations, Chemical Potentials and Thermodynamic Properties of Interoxide Compounds in the System Ba-Pb-O,” Mater. Sci. Eng., 1998, B52, pp. 134–44.
K.T. Jacob and K.P. Jayadevan: “System Sr-Pb-O: Phase Equilibria and Thermodynamics Using Solid-State Cells With Buffer Electrodes,” Chem. Mater., 2000, 12, pp. 1779–86.
K.T. Jacob, G.M. Kale, and G.N.K. Iyengar: “Chemical Potentials of Oxygen for Fayalite-Quartz-Iron and Fayalite-Quartz-Magnetite Equilibria,” Metall. Trans. B, 1989, 20B, pp. 679–85.
K.T. Jacob and J.H.E. Jeffes: “Deoxidation of Liquid Copper: Effect of Phosphorous on Oxygen Activity,” Trans. Inst. Min. Metall., Sec. C, 1971, 80, pp. C181-C189.
K.T. Jacob and J.H.E. Jeffes: “Thermodynamics of Oxygen in Liquid Copper, Lead and Copper-Lead Alloys,” Trans. Inst. Min. Metall., Sec. C, 1971, 80, pp. C32-C41.
K.T. Jacob and C.B. Alcock: “Thermodynamics of CuAlO2 and CuAl2O4 and Phase Equilibria in the System Cu2O-CuO-Al2O3,” J. Am. Ceram. Soc., 1975, 8, pp. 192–95.
L.B. Pankratz: “Thermodynamic Properties of Elements and Oxides,” Bureau of Mines Bull. 672, U. S. Department of the Interior, 1982, p. 143.
I.S. Sukhushina and I.A. Vasilieva: “Thermodynamic Properties of Europium Oxides,” Russ. J. Phys. Chem., 1990, 64, pp. 1734–36.
K.T. Jacob, unpublished research, 2002.
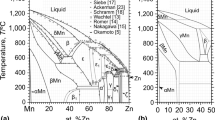
P.R. Subramanian and D.E. Laughlin: “The Cu-Eu (Copper-Europium) System,” Bull. Alloy Phase Diagrams, 1988, 9, pp. 342–47.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kisiel, R., Gasior, W., Moser, Z. et al. Phase relations in the system Cu-Eu-O and thermodynamic properties of CuEu2O4 and CuEuO2 . J Phs Eqil and Diff 25, 125–133 (2004). https://doi.org/10.1007/s11669-004-0005-0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11669-004-0005-0